
Healthcare technology firm Hyperfine Research is touting the results of a preliminary study that found that its portable point-of-care MRI scanner is viable and effective in a critical care environment.
The study is to be presented at the American Stroke Association's International Stroke Conference in Los Angeles on February 19 to 21 and comes in the wake of Hyperfine receiving 510(k) clearance from the U.S. Food and Drug Administration (FDA) for MRI head scans of patients two years of age and older.
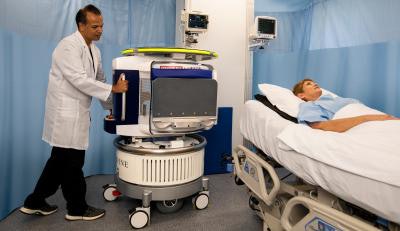 The Hyperfine point-of-care MRI scanner. Image courtesy of Hyperfine Research.
The Hyperfine point-of-care MRI scanner. Image courtesy of Hyperfine Research.In this study, 85 stroke patients (age range, 18 to 96) underwent scans with the low-field MRI scanner while in their hospital beds within seven days of symptom onset. Among the patients, there were 40 cases (46%) of ischemic stroke, 29 patients (34%) with intracerebral hemorrhage, and 17 people (20%) with subarachnoid hemorrhage.
The point-of-care scans took an average of approximately 30 minutes and most patients were able to complete the entire exam, showing that the device is well tolerated. Only five patients (6%) could not fit into the 30-cm opening, and six patients (7%) experienced claustrophobia, which prompted a halt to their scans.
"There's a lot of work to do," said senior author Dr. Kevin Sheth, chief physician in the division of neurocritical care and emergency neurology at Yale School of Medicine, in a statement. "However, we've cracked the door open for bringing this technology to any setting, anywhere."



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











