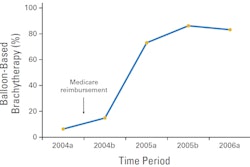
A study of more than 3,500 early-stage breast cancer patients reveals that women in the U.S. increasingly chose accelerated partial-breast irradiation (APBI) brachytherapy treatment over whole-breast radiation, despite the lack of clear clinical guidelines during the study period for APBI use.
APBI employs a balloon catheter inserted into the breast to deliver radiation directly to tumor sites. As an alternative to external-beam whole-breast radiation therapy, it offers a shorter, five-day course of treatment and is simpler to administer. The U.S. Food and Drug Administration (FDA) in 2002 approved the first APBI device for breast brachytherapy (MammoSite, Hologic).
Clinical trials on the effectiveness of APBI versus whole-breast radiation, however, are limited in number and still under way. The picture has been clouded further by the publication of results indicating that whole-breast radiation might have an edge over APBI in overall patient survival rates. Unfortunately, results from a head-to-head clinical trial comparing the two techniques may not be available for at least five years, and clinical guidelines on APBI from the American Society for Radiation Oncology (ASTRO) weren't published until 2009.
To get a better understanding of APBI use as the technology was being adopted, researchers from the University of Maryland Medical System examined patterns of care in the U.S. for MammoSite in early-stage breast cancer patients from 2002 to 2007. Patients were drawn from the U.S. National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database.
Dr. Zain Husain, a radiation oncology resident at the university, presented details of the study at the recent American Brachytherapy Society (ABS) annual meeting in San Diego. Husain's presentation was followed by an article published online June 12 in Brachytherapy.
Eligible patients were women 20 years and older who had histologically confirmed invasive carcinoma or ductal carcinoma in situ (DCIS). A total of 3,593 patients were included, ranging in age from 28 to 95, with a median age of 64 years. More than 80% of the cohort had invasive tumors (88.6%) that were smaller than 2 cm (80.9%) and estrogen-receptor positive (86.7%).
The Maryland team determined that APBI utilization increased nearly tenfold over the time period, from 163 cases in 2002 to 1,427 cases in 2007. Other trends identified included the following:
- No patients diagnosed with pure DCIS received breast brachytherapy in 2002, but by 2007 15.5% of DCIS cases were undergoing the procedure.
- Patients with nodal involvement getting breast brachytherapy steadily declined, from 12.3% of the total in 2002 to only 3.3% in 2007.
- The percentage of patients with tumors smaller than 1 cm gradually increased, from 36.2% in 2002 to 47.7% in 2007.
ASTRO's APBI guidelines
Although all the patients in the study underwent treatment prior to publication of the 2009 ASTRO consensus statement, Husain and colleagues reviewed the percentage who would have been considered suitable candidates if the guidelines had been in effect. They found that only 38.1% would be within the risk group who met all criteria for APBI, while 43.5% were in a "cautionary" group, only meeting some of the criteria for the procedure, and 18.3% were rated as "unsuitable."
The researchers had several opinions on what could account for the variance between APBI's rapid adoption rate and the subsequent ASTRO guidelines.
"That more than half of the patients fell into the cautionary or unsuitable groups can be interpreted as an aggressive adoption of new technology, or perhaps, staunch conservatism on the part of the consensus guideline creators, depending on one's perspective," they wrote.
The researchers went on to say that the ASTRO guidelines "seemed poised to significantly alter the utilization" of APBI. However, data are not yet available to measure any such impact.
Even though the current study did not include the time period after the ASTRO guidelines were issued, it provides baseline data for comparison by future utilization studies, according to Husain. Because calendar year 2008 data from the SEER database was just released in May 2011, several years will need to pass before the effects of the ASTRO guidelines can be analyzed.