
Conventional wisdom holds that when you hit the jackpot, you take your winnings and leave. It's also understood that you don’t take your windfall and use it to ante up in a completely new game.
Dr. Evan Unger, president and CEO of ImaRx Therapeutics in Tucson, AZ, doesn't seem to pay much heed to that type of thinking. In the past two years he's taken a lucrative buyout from DuPont Pharmaceuticals and parlayed it into a line of cutting-edge preclinical technologies.
ImaRx’s products can be broadly categorized as biotechnology that incorporates acoustically active microspheres, targeting vehicles, and proprietary formulations for delivering pharmaceuticals and gene therapy. Its focus is on energy-activated systems that use ultrasound to deliver drugs to treatment sites.
Naturally, taking big risks (and landing the venture capital to fund them) is a lot easier when one has a proven record of success. Unger has that too. Back in 1990 when he was a radiology professor at the University of Arizona in Tucson, Unger founded ImaRx Pharmaceuticals in order to develop medical imaging contrast agents.
The company signed its first joint-development agreement in 1995 to develop and market Definity, an ultrasound contrast agent for use in heart, liver, and kidney imaging. Definity uses microspheres that are 1 to 2 microns in size, and made from biocompatible polymers that reflect sound.
The microspheres are four times smaller than red blood cells, and several orders of magnitude more echogenic (reflective) than cellular material. A filter in the ultrasound transducer suppresses echoes of the transmitted wavelength, thereby focusing on the harmonic echoes created by the microspheres.
The result is increased contrast between the microspheres and the surrounding tissue. Use of the agent in testing provided enhanced visualization of the blood supply to organs, tissues, and tumors, and also revealed previously unseen vessels, Unger said.
Buoyed by the success of Definity in clinical testing, DuPont purchased the product, the patents, and the worldwide marketing rights for close to $40 million in cash and DuPont stock in October 1999. According to Unger, Definity has passed through all its clinical trials and is due for "imminent approval" from the Food and Drug Administration.
Bursting the bubble
The next product in the firm's preclinical development pipeline, MRX-408, is a clot-busting material dubbed SonoLysis. The technology utilizes microspheres coated with molecules that cause them to stick to thrombi. Ultrasound is then used to locate and "pop" the microspheres that have bound themselves to the thrombus. The energy released by the "popping" breaks up the thrombus and sweeps away the clot in small pieces. SonoLysis was successfully tested on animal models last year.
"The procedure requires only an intravenous injection -- no catheter, cutting, or guide wires," Unger said. "I envision the technology being administered by both cardiologists and interventional radiologists."
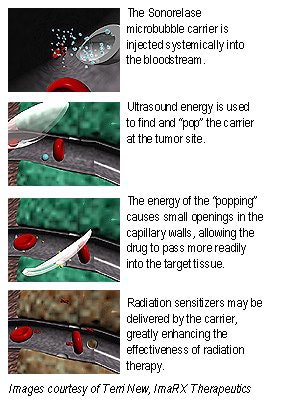
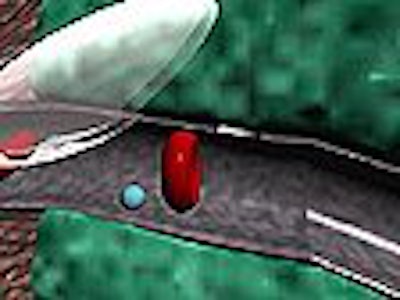
On the gene-therapy front, Unger sees great promise in utilizing microspheres as carriers to deliver genes directly to tumors. This technology, which ImaRx calls "Sonorelease," binds a gene (or a drug) to a microsphere. As with the SonoLysis product, ultrasound is used to locate and release the gene by popping the microsphere with acoustic energy.
The procedure may provide a safer and more effective alternative for gene-therapy delivery, according to the company. Currently, genes are delivered via a modified virus, which may cause allergic reactions in some patients. Even without microsphere delivery technology, ImaRx’s research has seen 10 to 1,000 times greater uptake in interleukin-12 in mice tumors when the product is combined with ultrasound energy.
The company expects the SonoLysis product to receive FDA approval in the next couple of years, as it is further along in preclinical trials.
"We’re slated to start clinical trials for SonoLysis late this year, and also expect to have Sonorelease approved in the next three or four years," said Unger.
Shaking the money tree
Funding a development-stage company from preclinical research to final clinical trials is not an inexpensive proposition. In December 2000, Unger showcased the company’s products at the Arizona Venture Capital Conference in Scottsdale, AZ. The following month, ImaRx successfully closed a fundraising round that brought in $6.3 million. The firm hopes to raise another $15 million to further speed product development.
On January 31, ImaRx signed a joint-venture agreement with Elan, a Dublin, Ireland-based pharmaceutical company. "With Elan, we now have the technical and financial resources to further advance the development of delivery systems," Unger said.
As part of the deal, Elan made an undisclosed equity investment in ImaRx, and provided a credit facility to fund the research and development of drug delivery technology for an oncology product. ImaRx will retain majority ownership of the venture.
Unger does not rule out the possibility of an initial public offering of stock at some point in the future, as well as additional joint ventures with equipment manufacturers.
"These products show the synergy that’s possible between device and drug companies when applications (ultrasound and microspheres) are combined," he said. "We’re creating the possibility of imaging-source therapy."
By Jonathan S. BatchelorAuntMinnie.com staff writer
February 12, 2001
Relating Reading
P53 gene therapy shows promise in ovarian cancer, September 22, 2000
New interventions show promise in battling head and neck cancers, August 11, 2000
Gene therapy: New frontier for interventional radiology?, July 5, 2000
Click here to post your comments about this story. Please include the headline of the article in your message.
Copyright © 2001 AuntMinnie.com