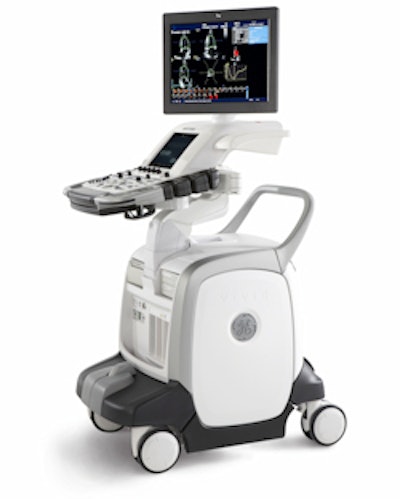
GE Healthcare of Chalfont St. Giles, U.K., is launching a pair of new echocardiography scanners at next week's American Heart Association (AHA) meeting, including a new system optimized for 4D cardiac scanning.
Vivid E9 is a new system designed from the ground up for 4D (3D with motion) echocardiography, according to the company. The scanner was designed with GE's Accelerated Volume architecture, with extra processing power for handling larger data volumes and a more powerful beamformer, according to the company. GE estimates that 4D imaging requires processing eight times more data than conventional techniques.
 |
| GE's new Vivid E9 echo scanner. |
Additional functionality for the system includes 4D Virtual Store, which uses image pointers to help users better manage the large datasets 4D imaging creates. In addition, Vivid E9 is the first GE scanner able to perform true 4D stress echo, the company said. Using new Scan Assist technology, Vivid E9 users can acquire single-cycle or multicycle full volumes in any variation of traditional, multidimensional, or full-volume views. Vivid E9's templates for both exercise and pharmacologic stress tests all are customizable.
Vivid E9 also includes a new M5S matrix-array transducer and matches it with the company's single-crystal technology. GE believes this provides better endocardial definition, texture, and crisper valve delineation across a wider range of frequencies than with traditional transducers.
Vivid E9 will carry a price point 20% to 30% higher than the Vivid 7 scanner and will be targeted at facilities that want to incorporate 4D imaging into their practice. Vivid 7 will remain in the company's product line as a workhorse echo scanner for facilities that only perform 4D occasionally. Commercial deliveries of Vivid E9 will begin at the end of the year.
Another new scanner being introduced at the AHA show is Vivid q, a new offering in GE's compact echocardiography ultrasound line. The scanner is similar to the Vivid i compact system, but with new technologies migrated from the Vivid 7 scanner, such as an M4S matrix-array transducer.
Vivid q also includes GE's AutoEF automated ejection fraction calculation and other quantification features. Meanwhile, a tissue synchronization imaging (TSI) mode with quantitative analysis represents a parametric imaging mode that enables physicians to see quantitative information in moving 2D images.
Finally, Vivid q will support GE's intracardiac echo (ICE) technology, based on the Acuson AcuNav 10F ultrasound catheter. ICE provides real-time image guidance and visualization of anatomical structures to help clinicians increase their confidence when performing electrophysiology and interventional cardiology procedures. GE in October inked a deal with Biosense Webster of Diamond Bar, CA, to gain access to the technology.
Vivid q will carry a price point 10% to 15% higher than Vivid i and will be targeted at the hospital market, GE said.
Related Reading
Road to RSNA, Ultrasound, GE Healthcare, November 5, 2008
Road to RSNA, MRI, GE Healthcare, October 31, 2008
GE completes Vital Signs buy, October 31, 2008
Road to RSNA, CT, GE Healthcare, October 29, 2008
Road to RSNA, Women's Imaging, GE Healthcare, October 28, 2008
Copyright © 2008 AuntMinnie.com



















