
AuntMinnie.com presents the eighth in a series of columns on the practice of ultrasound from Dr. Jason Birnholz, one of the pioneers of this modality.
Fellow UltraSounder,
Over the centuries, medical people have been marvelously inventive, getting the most out of whatever tools and techniques they had. I remember being taken by the story of Leopold Auenbrugger, who essentially developed a technique that is the true technical ancestor of ultrasound in 18th-century Austria.
 |
| Dr. Jason Birnholz. |
In ultrasound, we replace the tapping finger with a sound emitter and the feeling or hearing of reverberant waves with a piezoelectric sensor. Voila, A-mode ultrasound, the first implementation, followed pretty soon thereafter with area scanning and ultrasound image generation.
Inventive as we are, once something seems to work well enough in a clinical setting, some of us keep on doing the same thing, no doubt getting faster at the task from practice, but never inquiring if it's still the best that we can do. This is a major problem in clinical ultrasound because equipment performance has been improving both in quantum leaps and at a slower, steadier pace for essentially all of its 50-year history. During that time, there has also been a vast increase in our understanding of disease and our capabilities for effective therapy.
This lag between capabilities and practice seems to be an everyday occurrence in obstetrical ultrasound. Figure 1 is a September 2012 portrait of a 20-week fetus (19.6 weeks gestational age). Some of you will likely have newer and better images already. One immediate issue is the following: Given this level of imaging, should we still be following 1970s or earlier precepts of practice for any part of the ultrasonic fetal evaluation?
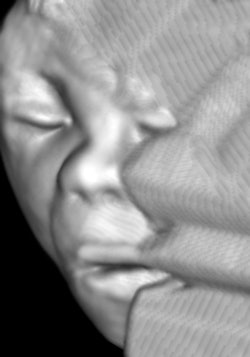 Figure 1: 3D image of a healthy 19.6-week male fetus. All images courtesy of Dr. Jason Birnholz.
Figure 1: 3D image of a healthy 19.6-week male fetus. All images courtesy of Dr. Jason Birnholz.
This is the core issue of this article via a sidelong glance at some aspects of fetal "biometry." I love that term; it's so precise, yet so grand for what it involves. Fetal measurements are a boring topic, but boring below the surface may yield us a few practice nuggets.
Gestational age
Determining gestational age (GA) is part of every ultrasound exam. We really want to know conceptual age, or time since conception in days or weeks. GA is just conceptual age plus two weeks, i.e., you start at two and, on average, finish at 40. This preserves the traditional form of menstrual dating, which I believe also dates to the mid-18th century. I haven't asked a patient for menstrual dates in years, since ultrasound is so good at the task.
If the date of conception is known, of course, that information is pure gold. In in vitro fertilization (IVF) patients, one uses egg-retrieval day, which is when conception happens (with frozen embryos, you subtract the conceptual age, often five days, from the implantation date). Knowing the GA as exactly as possible is important for scheduling repeat cesarean sections; more importantly, for avoiding postmaturity entirely; and most importantly, for establishing a moving reference for evaluating growth and milestones of development.
Basic measurement and calculation issues
There is a strangely retro practice in some places of referring to gestational age in weeks and days. This seems to me a peculiar affectation because it makes it necessary to use a spreadsheet or programmable calculator for converting that info into and out of simple decimal format for calculations. Of course, to each his own, if it works. Perhaps we will also return to the apothecary system, such as prescribing two drachms of mistletoe tincture, but I suspect not.
Estimating GA is a kind of forensic problem, usually done by correlating one or more ultrasonic measurements with a reference series of measurements that has been validated for the same (fetal) population. Because the process involves deciding what the best estimate might be, estimation of gestational age is an interpretation, i.e., it is a form of diagnosis, which has legal consequences in alleged negligence cases.
You will all have learned about the multiple factors that are involved in ultrasonic biometry in general. There are just a few things I would like to emphasize. Measurements made in depth depend on propagation velocity setting, typically 1,540 m/sec, but adjustable in the newest equipment. However, measurements made with a linear array along the axis of the probe are "absolute," depending only on the number and spacing of the elements.
With small-footprint probes scanning in a sector format, the precise measurement is angular deviation, not distance. A further problem, beam refraction, may result if the path contains a lot of fat (lower velocity) or the placenta (1° to 2° warmer than the background, higher velocity). Curved arrays can be problematic, but it is safe enough to assume that they are not far off from linear arrays near the middle of the scanning field. Also, it is important to maximize the pixels/tissue millimeter ratio for the display to decrease measurement error -- that is, fill the screen with whatever it is that needs to be measured.
In clinical practice, the utility of a measurement is degraded by operator and equipment errors and by inherent biological variation for each measurement variable. A lot of the early works on fetal measurements naively or optimistically assumed that the main limitation was in inherent variation, but the truth is that operator error outweighs biological variation (for linear fetal measurements) by a ton. Make that 10 tons.
Area and perimeter measurements are more error prone than simple diameters. Finally, it is a good practice to make each individual measurement a few times; I like three to five times for routine cases. But you don't want to average those results! Just look at the values that cluster and throw away all outliers. You never want to average good and bad data.
Biparietal diameter
Biparietal diameter (BPD) is the classic ultrasound determinant of GA that textbooks and courses all seem to want to preserve. Ever wonder how it came about? I am going to indulge in some history dredged from memory, so I will likely get a lot of attributions wrong. If you have an interest in this area, the landmark work on fetal measurements and the history of the subject were by Scammon and Calkins in the 1920s.
Various transverse diameters of the head can be traced back to at least the 19th century, usually with a giant caliper that looked like ice tongs. Embryologic and fetal measurements appear in the works of the great embryologists at the start of the 20th century, such as His, Mall, and Streeter, although they were most concerned with early development. Pretty soon after radiography became widely used, x-ray pelvimetry built upon pathology-based measurements of BPD as a part of the prediction or exclusion of cephalopelvic disproportion. BPD was around long before ultrasound came along.
The earliest implementation of ultrasound was the A-mode, mainly used for identifying shifts of the intracranial "midline" from a subdural hematoma after trauma. I think the first reports of using A-mode for BPD over a palpable head near-term was in the late 1950s from Donald and Brown in Glasgow and Kohorn in Baltimore. Stuart Campbell in London was instrumental in extending this throughout the second and third trimesters for GA determination in the 1960s, early in the B-mode experience. Every country has its famous obstetrical ultrasound pioneer who contributed massively to the development of this most widely used of all ultrasound applications; my opinion is that Stuart Campbell did more than anyone to establish the field and guide its development worldwide.
Getting back to the theme, BPD became the norm when the cranium was pretty much the only thing that one could see easily with the early manual scan equipment. Using it was the smart thing to do, but it was not that someone with an overview of fetal growth and development said, "Pick this because it is the best measure biologically." To be sure, it's not bad, or we wouldn't have exerted so much effort to preserve the BPD and refine it by specifying exact protocols for measurement and by compensating for shape changes with additional diameters, area, or circumference estimates.
From a sampling standpoint, the cranium grows in volume, which is cubic, from which the linear BPD picks off one dimension. Even forgetting that the cranium is a kind of hemiellipsoid with some shape flexibility, any relationship between BPD and GA (time) cannot be a simple linear one.
Long bones, such as the femur, are a much better choice for GA estimation. The largest magnitude growth change is in length and acoustic contrast there is high, making this an easy, precise measurement with a linear array. Femur length was introduced in the 1980s, possibly by John Queenan and popularized by John Hobbins. Figure 2 shows femur length versus gestational age in a clinically mixed group of some 3,000 well-staged fetuses without structural anomalies or aneuploidy.
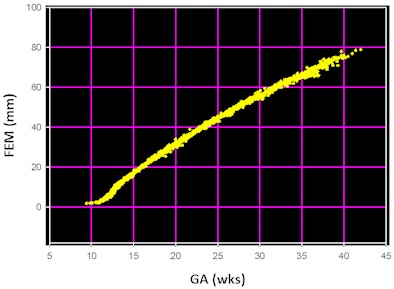 |
| Figure 2: Femur shaft length versus gestational age in 3,182 well-staged euploid fetuses without anomalies, referred from a variety of practices to Diagnostic Ultrasound Consultants in Oak Brook, IL. |
From the time that shaft length becomes the dominant mode of growth at 12 weeks GA, the incremental pattern is nearly linear and there is no significant change in data dispersion through gestation. That is, it's usable until the end.
A special feature of early pregnancy
One of the enduring works of early ultrasound is Hugh Robinson's determination of gestational age from embryonic length, popularly referred to as crown-rump length from the 1970s manual scan era. To be sure, embryos could not be visualized very early in pregnancy then. The reason this works so well in the mid to late first trimester is that embryos are relatively flat, the numerically dominant growth parameter is in length, and there is high contrast between the embryo and fluid within the bubble formed by the amnion, sort of like the situation with femur length after 12 weeks.
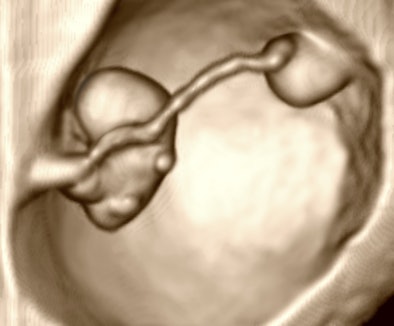 |
| Figure 3: 3D image of a 13.2-mm embryo. Heart rate from a four-second Doppler recording was 149.58. Stage is 7.6 weeks. |
The embryo has a submillimeter length when first "seen" around 24 days after conception, when effective measurements are difficult with most systems. By the time an embryo can be seen, the heart has started to beat. There have been a number of studies showing that from whatever starting point is taken, heart rate increases to a peak of around 190 beats per minute near 9.5 weeks GA, after which it drops and stabilizes, typically in the 140s until the later third trimester.
A tip about fetal heart rate determination: Use a tracing with obvious deflections during parts of each cardiac cycle, and for whatever length of recording you have, calculate rate from the time span and the number of beats between the first and last beat of the tracing. Too many people do the interval between two beats, because this may be read-out directly. This is a sure way to maximize error.
We always use high-frequency Doppler (the higher the better, as backscatter signal amplitude is proportional to the fourth power of frequency), and we record from two to five seconds. Even this short a tracing can yield values within plus or minus one beat per minute over the physiologic heart rate range of early pregnancy.
Some elegant studies have indicated that pacemaker electrical activity is initiated through intracellular calcium oscillations mediated by an inositol 1,4,5-triphosphate cycle (Méry et al, Molecular Biology of the Cell, May 1, 2005, Vol. 16:5, pp. 2414-2423) and that heart rate is tightly programmed by the progressive deployment of ryanodine receptors (Huang-Tian et al, Proceedings of the National Academy of Sciences, July 9, 2002, Vol. 99:2, pp. 9225-9230).
What I found in about 950 embryos younger than 9 weeks or at about 22-mm crown-rump length (CRL) was that cardiac motion was visible from about 5.6 weeks GA or 0.6-mm CRL and that Doppler tracings could be obtained even then. All of these were referred cases, typically with spotting or some worry about threatened abortion, although some were sent because of uncertain staging.
All we required was that the pregnancy be continued through at least 20 weeks GA. The heart rate increased rapidly from 66 beats per minute at 5.6 weeks to about 100 beats per minute at 2.4 CRL or 6 weeks GA. We discarded these cases, which left 854 from 6 to 9 weeks when heart rate increased almost linearly. Let me share a simple formula for that interval that had an r2 = 0.898 for these data: GA = 3.9429741 + 0.0019595647 FHR1.5.
This seems to be the very best way to stage an embryo, getting accuracy to about a day, all of which derives from the numerical range for heart rate and the precision with which it can be determined. This age range is the critical period of embryogenesis of the heart, starting when the heart is just a tube.
The real message
This embryonic heart rate business may seem to be some kind of trivial exercise. After all, for the typical uses of GA, is such accuracy necessary or even worthwhile? Well, the bottom line (which this almost is) is that even minimal discrepancies between early embryonic heart rate and GA or CRL or an abnormal heart rate increment between two closely spaced exams seems to be a very sensitive and specific predictor of a poor outcome.
When you use pulsed Doppler, there are other markers of diminished pump function, which we know. In our experience, concordant heart rate and staging between 6 and 9 weeks GA means a 98% or greater likelihood that the pregnancy will continue, and therein may be its best usage in practice.
Dr. Jason Birnholz is a graduate of Johns Hopkins School of Medicine and did his diagnostic radiology residency at Massachusetts General Hospital. He was awarded an advanced academic fellowship from the James Picker Foundation and has been a professor of radiology and obstetrics.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnie.com, nor should they be construed as an endorsement or admonishment of any particular vendor, analyst, industry consultant, or consulting group.



















