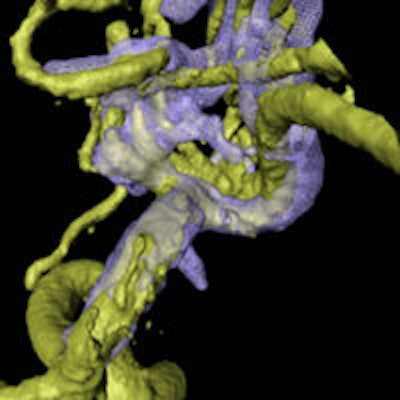
Combining rotational digital subtraction angiography (DSA) with 3D ultrasound creates a powerful pair for guiding aneurysm surgery when images are dynamically coregistered during procedures, according to a new study by German researchers and published in PLOS One.
Intraoperative coregistration of rotational DSA (rDSA) with intraoperative 3D ultrasound (3D-ioUS) allows immediate visualization of vessels beyond the microscopic field, along with a parallel assessment of blood velocity, the aneurysm, and the complete configuration of the vascular tree. The new technique heralds an important improvement in cerebrovascular interventions, wrote researchers from the Dresden University of Technology in Germany.
"The immediate intraoperative coregistration of 3D-ioUS and rDSA of cerebral aneurysms conveys one of the many benefits of US in the neurosurgical operating theater and shows that although the ultrasound data volumes were difficult to interpret on their own, the coregistration to rDSA images is important for interpretation," wrote Dr. Dino Podlesek and colleagues Ute Morgenstern, Dr-Ing; Dr. Gabriele Schackert; and Matthias Kirsch, PhD (PLOS One, March 24, 2015).
Preoperative planning problems
Previously at the institution, all intracranial aneurysm patients with surgeries being planned underwent CT angiography, MR angiography, and DSA. In the experience of the authors, however, the interpretation of preoperative diagnostic imaging, even using multiple modalities, does not always match the real intraoperative situation in patients.
Aneurysm surgery has three main goals: complete clipping of the intracranial aneurysm, preservation of the vascular tree, and identification of vessels beyond the visual field, the authors wrote. Surgeons aren't always successful in completely clipping the aneurysm; a study by Le Roux and colleagues found aneurysm remnants in nearly 6% of patients treated by clipping at postsurgical angiography.
"Intraoperative ultrasound (3D-ioUS) might improve this uncertainty since it is not limited to the visual field and allows for online imaging and real-time update of images, respectively," Podlesek and colleagues wrote. "The purpose of our study was to evaluate the usability, the accuracy, and the applicability of intraoperatively applying volume-based registration of 3D-ioUS in comparison with preoperative rDSA."
The study analyzed 3D-ioUS in 39 patients (13 men, 26 women; median age 57 years, range 35-80 years) with 59 saccular cerebral aneurysms to visualize the aneurysm intraoperatively and the nearby vascular tree before and after clipping, the authors explained. The clinicians selected 43 of the 59 aneurysms for neurosurgical clipping and intraoperative ultrasound imaging, treating the rest with coiling.
The study team performed simultaneous coregistration of preoperative subtraction angiography data with 3D-ioUS to verify the anatomical assignment. Setup and completion time was less than 10 minutes. Consecutively with the procedure, the clinicians imported 3D-ioUS datasets to a laptop computer with a stereoscopic display; images were coregistered with preoperative rDSA, which produced a rapid 3D display of the coregistered vascular tree.
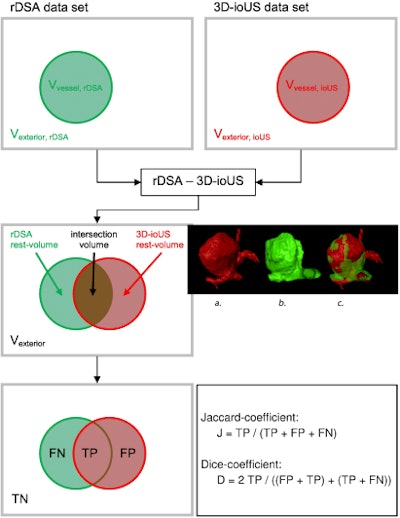 Pattern of the evaluation of registration results by overlapping the rDSA and the 3D-ioUS (TN: true negative, FN: false negative, TP: true positive, FP: false positive). All images courtesy of PLOS One (doi:10.1371/journal.pone.0121345.g006).
Pattern of the evaluation of registration results by overlapping the rDSA and the 3D-ioUS (TN: true negative, FN: false negative, TP: true positive, FP: false positive). All images courtesy of PLOS One (doi:10.1371/journal.pone.0121345.g006).Micro-Doppler measurements were routinely performed both before and after clip placement with the aid of a 16-MHz conventional micro-Doppler probe by applying probe directly to the visible vessels. Near-infrared indocyanine green videoangiography also was performed in a few selected patients.
Thirty-nine patients with anterior, middle cerebral, and internal carotid artery aneurysms underwent high-resolution power Doppler 3D imaging (Voluson 730, GE Healthcare) to visualize the aneurysm and the vascular tree before and after clipping, the authors wrote. The unit was equipped with a curved linear-array transducer and contained a mechanism to pivot the ultrasound crystals during data acquisition, while the probe was attached to a pneumatic arm to minimize motion artifacts, they wrote.
Hardware and software visualization tools included a notebook computer with autosteroscopic display, which enabled immediate 3D impressions of vascular morphology. A 3D mouse was used to support navigating and maneuvering objects in 3D space. The Amira software application (FEI) was used for digital 3D planning and editing.
In all, intraoperative ultrasound detected 35 of 43 aneurysms (81%) in 39 patients, the study team reported. Thirty-nine intraoperative ultrasound measurements were matched with rDSA and were successfully reconstructed intraoperatively. The volume overlap between rDSA and 3D ultrasound was compared to evaluate the similarity after registration. The volume of interest was centered on the aneurysm in both datasets and cropped to fit the aneurysm, including the aneurysm neck. Finally, counts for true positives, false positives, and false negatives of the overlap were calculated for each registration.
In seven patients, the aneurysm was only partially visualized by 3D ultrasound or was outside the field-of-view. Postclipping intraoperative ultrasound was obtained in 26 patients -- successfully in 18 patients (69%) despite some clip-related artifacts. Finally, the overlap between 3D-ioUS volume and preoperative rDSA aneurysm volume resulted in a mean accuracy of 0.71 (Dice coefficient).
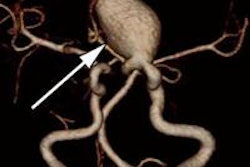
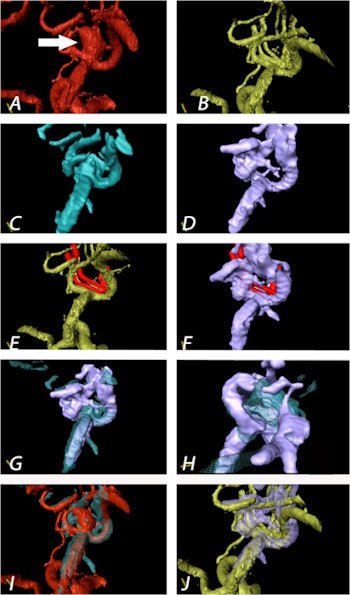 Aneurysm of the medial cerebral artery bifurcation indicated by the white arrow. Precise aneurysm neck visualization in 3D-ioUS and clip segmentation for virtual planning of clipping procedure. A: preclipping rDSA (red); B: postclipping rDSA (green); C: preclipping 3D-ioUS (blue); D: postclipping 3D-ioUS (pink); E: postclipping rDSA with segmented clips (red); F: postclipping 3D-ioUS with segmented clips (red);G-H: coregistration of C+D; I: coregistration of A+C; J: coregistration of B+G.
Aneurysm of the medial cerebral artery bifurcation indicated by the white arrow. Precise aneurysm neck visualization in 3D-ioUS and clip segmentation for virtual planning of clipping procedure. A: preclipping rDSA (red); B: postclipping rDSA (green); C: preclipping 3D-ioUS (blue); D: postclipping 3D-ioUS (pink); E: postclipping rDSA with segmented clips (red); F: postclipping 3D-ioUS with segmented clips (red);G-H: coregistration of C+D; I: coregistration of A+C; J: coregistration of B+G."The result of coregistration was demonstrated to two ultrasound-experienced vascular neurosurgeons who subjectively evaluated the coregistration results intraoperatively," Podlesek and colleagues wrote. "Their assessment was excellent with coregistered identification of the vascular structures."
Intraoperative semiautomated coregistration of 3D intraoperative ultrasound with preoperative rDSA "combines the advantages of ultrasound, namely speed, noninvasiveness, and visualization beyond the visible field, with an improved anatomical interpretation of the coregistered 3D-US images," the team wrote. "Using standard visualization tools, it is possible to simulate and evaluate the intraoperative strategy when the anatomical structures were afflicted by brain shifting and other changes concerning the anatomical discrepancy to the preoperative data."
The combined technique overcomes many of the limitations that each modality has on its own in guiding surgery, the researchers concluded. In the future, they hope to explore new clinical applications for the approach.
"Further developments should combine power 3D-ioUS integrated into a neuronavigation system for identification and reduced misinterpretation of ioUS data due to brain shift, in perspective allowing for transcranial 3D color-coded Doppler and the possibility for blood flow velocity quantification in 3D," they wrote.