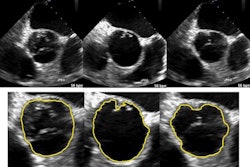
A new automated hip segmentation algorithm that uses 3D ultrasound data produced accurate segmentations of the acetabulum in infant hips, according to a study in the International Journal of Computer Assisted Radiology and Surgery. The technique could improve the diagnosis of infants with developmental dysplasia of the hip (DDH).
When they tested the algorithm on 51 infant hip models, the researchers found excellent agreement between semiautomatic and manual segmentation volumes. Semiautomatic contouring was also faster and more reliable than manual segmentation, according to lead author Abhilash Rakkunedeth Hareendranathan, a research fellow in the radiology department of the University of Alberta in Edmonton, Canada.
"We were able to generate surface models of the acetabulum from 3D ultrasound with minimal user interaction," he wrote in an email to AuntMinnie.com. "Various clinically useful indices were calculated from these models and used in DDH classification."
A 'formidable challenge'
Developmental dysplasia of the hip can lead to premature osteoarthritis if left untreated. Even though 3D ultrasound is a feasible radiation-free alternative to CT for hip imaging, automated techniques for hip joint segmentation are rare due to serious technical challenges. For example, the presence of speckle noise in echocardiography scans means that threshold-based approaches common in CT can't be used for ultrasound-based segmentation schemes, according to the authors.
 Abhilash Rakkunedeth Hareendranathan from the University of Alberta.
Abhilash Rakkunedeth Hareendranathan from the University of Alberta.Automatic segmentation of anatomical structures is a "formidable challenge" owing to high levels of image noise, blur, and artifacts that occur with ultrasound, they wrote (Int J Comput Assist Radiol Surg, June 20, 2015).
Not surprisingly, most work in hip segmentation has been with CT. Among the ultrasound techniques tried in previous research, Luis-Garcia et al in 2006 calculated the relative geometry of the acetabulum and the femoral head with 3D ultrasound by adjusting a parabolic surface for the acetabulum and a sphere for the femoral head, Hareendranathan and colleagues wrote. The method takes into account both the gray level and texture information in the ultrasound image. However, intensity variations in the image such as speckle noise were found to adversely affect segmentation.
Quantifying the acetabular shape is critical in diagnosing DDH using ultrasound, the authors wrote. However, current established techniques still fall short: For example, the Graf technique, a quantitative hip classification system for DDH diagnosis, is burdened by high interobserver and interscan variability.
In previous work, the authors showed that acetabular surface models can be generated from 3D ultrasound with high accuracy. But the method relied on manual segmentation, which was cumbersome and time-consuming, primarily due to the complexity of the hip anatomy, they wrote.
For this project, the group developed a semiautomated segmentation technique to rapidly generate 3D acetabular surfaces and classify the acetabulum based on the acetabular contact angle (ACA) derived from the models, Hareendranathan and colleagues wrote. That core calculation, the acetabular contact angle, represents the angular separation between the acetabular roof and the lateral iliac wall and reflects the underlying hip geometry, they explained; ACA is reduced in dysplastic hips.
The study tested the feasibility and reliability of the technique, and compared it with the manual segmentation method.
Looking past ultrasound's blind spots
The authors approached acetabular segmentation as a graph-search problem -- specifically, a path-finding problem, where the algorithm finds the optimal path through user-generated seed points on a graph.
"In the graph-search approach, every pixel in the image is treated as a graph vertex," Hareendranathan told AuntMinnie.com. "These vertices are connected in such a way that the shortest path on the graph is along the acetabular roof. The advantage of graph search is that it can 'jump' across discontinuities, such as portions of the bone that are obscured by [acoustic] shadowing. This is well-suited to ultrasound."
Dynamic programming techniques are used to generate the seed points. The algorithm then interpolates the slice contours over the 3D volume to generate the surface model. Finally, a 3D acetabular contact angle is calculated using the normal vectors of the surface model.
The technique was inspired by the so-called intelligent scissors algorithm developed by Mortensen et al, which also treats segmentation as a graph-search problem.
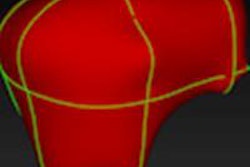
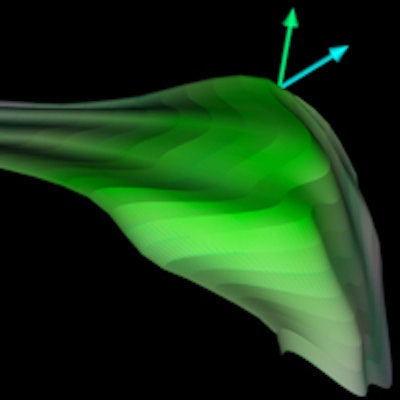
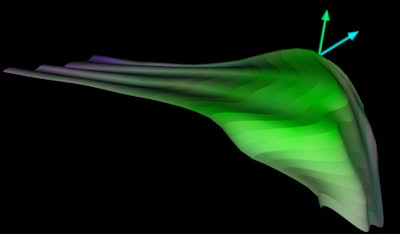 Acetabular model generated from 3D ultrasound. Image courtesy of Abhilash Hareendranathan.
Acetabular model generated from 3D ultrasound. Image courtesy of Abhilash Hareendranathan.Close to manual segmentation
Hareendranathan and colleagues tested their algorithm on 51 hip ultrasound volumes from 42 patients with hips ranging from normal to dysplastic.
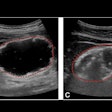
The contours generated by the segmentation algorithm closely matched those obtained in manual segmentation, the researchers found. The average root-mean-square error between the semiautomated and manual segmentations was 0.28 mm (or 1.1 voxels with two node points); when the apex point of the contour was used as the third seed point, the average dropped to 0.24 mm (0.9 voxels).
"The semiautomatic algorithm gave visually acceptable results on images with moderate levels of noise and was able to trace the boundary of the acetabulum even in the presence of significant shadowing," the authors wrote.
Semiautomatic contouring was also faster than manual segmentation, at 37 seconds versus 56 seconds for manual segmentation per scan. The method produced high-fidelity surface models, they wrote, and it is well-suited for ultrasound bone segmentation in general, as the effect of image noise is minimal, added Hareendranathan.
The acetabular contact angle calculated from the model "showed a clear distinction between normal and dysplastic hips and was able to classify the subjects into the three categories," the group wrote. The ACA also correctly classified most borderline cases as normal -- findings that were eventually shown to be correct.
Finally, the algorithm also improved the repeatability of the ACA calculation, revealing interobserver variability of just 1.4° ± 0.9° and intraobserver variability of 1.4° ± 1.0°.
Graph-based automatic segmentation offers a fast and reliable way to delineate the 3D surface and shape of the acetabulum from 3D ultrasound volumes of the hip, the authors concluded. Even its treatment of noisy data with missing echogenic boundaries is robust, because the technique does not rely on contour evolution. The technique could also be generalized to other anatomic regions and even modalities.
Although the researchers applied the automated segmentation technique to the infant acetabulum in 3D hip ultrasound, the technique could be generalized for any 3D or dynamic 2D ultrasound dataset, Hareendranathan and colleagues concluded. The authors plan to expand the project for their next study.
"We [will] be extending this technique by developing more 3D indices and testing whether they are clinically useful in prospective and multicenter studies," Hareendranathan said.