Philips HealthCare parent Royal Philips has received U.S. Food and Drug Administration (FDA) clearance for its new Mini TEE ultrasound transducer.
X11- 4t Mini 3D TEE is intended for cardiovascular ultrasound imaging and was designed to be smaller to meet unmet needs in pediatric patients (as small as 5 kg), adults at risk of complications, and in complex intensive-case-unit (ICU) cases, in which the probe for the company’s 3D TEE transducer was too large, Philips said.
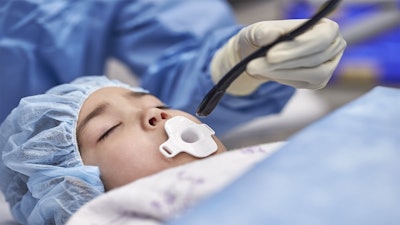 A clinician using the Mini TEE 3D transducer on a pediatric patient.Image courtesy of Royal Philips
A clinician using the Mini TEE 3D transducer on a pediatric patient.Image courtesy of Royal Philips
The Mini Live 3D TEE transducer operates with the same hand control, procedure navigation techniques, and workflow as with the company’s EPIQ cardiac ultrasound systems and as a result, echocardiographers may require minimal additional training on the new transducer, the company noted. In addition, the transducer is compatible with Philips’ premium cardiology ultrasound portfolio, including the EPIQ CVx and EchoNavigator image-guided therapy systems.
The transducer is scheduled for commercial availability in 2024 and is pending CE mark in Europe, Philips added.



















