After years of development, women's healthcare vendor Hologic of Bedford, MA, today received approval from the U.S. Food and Drug Administration (FDA) for its Selenia Dimensions 3D system, the first x-ray mammography device that provides 3D digital breast tomosynthesis images for breast cancer screening and diagnosis.
Dimensions 3D produces 3D images to reveal the inner architecture of the breast without distortion caused by tissue shadowing or density. The system uses a panning gantry head that moves in an arc around the patient, collecting multiple tomographic slices that can be reconstructed into 3D volumes.
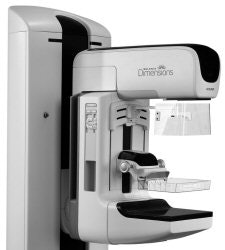 |
| Selenia Dimensions features a gantry modified for tomosynthesis. Image courtesy of Hologic. |
The product is already available commercially in more than a dozen markets outside the U.S., including Europe, Latin America, and Asia. Systems are also installed in Canada and Mexico.
Hologic's own data submitted to the FDA indicated that Dimensions 3D had a statistically significant impact on sensitivity compared to 2D mammography. The technology also reduced the recall rate for patients who did not have cancer.
As part of the approval process, the FDA reviewed results from two clinical studies where board-certified radiologists were asked to review 2D and 3D images from more than 300 mammography exams.
In both studies, radiologists viewing both the 2D and 3D images obtained a 7% improvement in their ability to distinguish between cancerous and noncancerous cases compared with viewing 2D images alone.
While the combination of the 2D and 3D images approximately doubled the radiation dose patients received, the system improved the accuracy with which radiologists detected cancers, decreasing the number of women recalled for a diagnostic workup.
"Physicians can now access this unique and innovative 3D technology that could significantly enhance existing diagnosis and treatment approaches," said Jeffrey Shuren, MD, director of the FDA's Center for Devices and Radiological Health, in a written statement.
Hologic executives also welcomed the approval, which came after the FDA reexamined how it reviewed full-field digital mammography systems.
"We are extremely proud to be the first company to receive FDA approval of a 3D digital mammography system and to offer women this ground-breaking, superior imaging technology," said Rob Cascella, Hologic president and CEO. "We believe tomosynthesis has the potential to change how screening and diagnostic mammography is performed, and over time will prove invaluable to the earliest possible detection of breast cancer and in the reduction of unnecessary diagnostic interventions."
Dimensions 3D software can be purchased as an option on existing Selenia Dimensions 2D systems. Enabling the 3D capability on a 2D system involves a software key and adjusting a PC board setting, and it does not require new hardware, according to the company.
By Wayne Forrest
AuntMinnie.com staff writer
February 11, 2011