A U.S. Food and Drug Administration (FDA) panel voted 9 to 1 on October 24 to recommend approval for a new imaging mode on the Selenia Dimensions 3D digital breast tomosynthesis (DBT) system from Hologic that creates synthesized 2D images from 3D data.
The recommendation specifically addresses the use of synthesized 2D images generated by Hologic's C-View software module in place of traditional 2D images when used in conjunction with 3D mammography, Hologic said. C-View is designed to enable tomosynthesis to produce 2D mammography images without the higher radiation dose typically involved in combined 2D/3D studies.
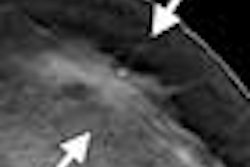
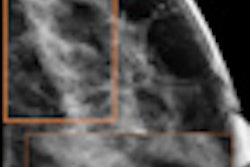
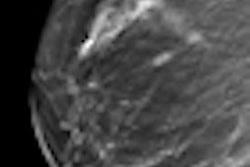
Since winning FDA approval in 2011, tomosynthesis has been promoted as a technology that could improve breast screening by collecting a range of data slices acquired as the mammography system's tube head pans around the breast. These slices are typically reconstructed into dynamic 3D volumes that enable radiologists to see around superimposed tissue in the breast.
However, radiologists still need a conventional 2D study as part of a comprehensive breast screening exam. Without C-View, tomosynthesis users were required to perform a separate 2D study along with the 3D, called combination-mode imaging, which delivers more radiation dose to the patient. Instead, C-View allows the 2D exam to be derived from the 3D tomosynthesis data by creating synthesized 2D images that can be used in conjunction with the tomo images.
Hologic's premarket approval (PMA) application for C-View describes the technology as a new operational mode that provides better clinical performance than traditional 2D mammography; however, it does so with a lower dose than combination-mode imaging, and with similar scan time, the same user interface, and no change in patient positioning, according to Hologic.
This mode is similar to combination-mode imaging, except that the 2D image the radiologist reviews is synthesized from the tomosynthesis dataset and does not involve additional radiation dose beyond that of the tomosynthesis scan, which is approximately 45% less than the present screening exam, which involves both 2D and 3D studies, Hologic said.
The synthesized 2D image is a maximum intensity projection (MIP) created from collapsing the 3D image set to a single 2D image; the synthesized 2D images can be displayed together with the 3D tomosynthesis images and will have "C-View" incorporated into the pixel data to alert users that they are not full-field digital mammography (FFDM) images, according to Hologic.
Selenia Dimensions 3D is currently approved for breast cancer screening and diagnosis, and the screening exam can consist of either full-field digital mammography (FFDM) alone or in combination with DBT, Hologic said.
FDA cautious
The FDA met with the Radiological Devices Panel because using synthesized 2D images with DBT as a new exam option for breast cancer screening will affect how mammography is performed, as FFDM images will not be acquired, FDA said.
"Currently, FFDM is the standard of care for breast cancer screening, and DBT was originally approved for the use with FFDM," the agency wrote in its presentation rationale. "While the synthesized 2D images are intended to resemble the FFDM images, Hologic is not claiming that the synthesized images are equivalent to FFDM images. The image quality of the synthesized 2D images alone was not directly compared to FFDM. The study design reflects the indication for the synthesized 2D images to be used with DBT."
The FDA asked the panel to discuss aspects of performing mammography that should be considered in training and/or labeling when FFDM images aren't acquired during the screening exam; to consider any specific concerns with respect to Hologic's retrospective study design; and to comment on the radiation risk of the Selenia 3D system with C-View.
C-View synthesized 2D images will not replace traditional 2D images, Hologic said.
"[We have] no plans to discontinue the combo-mode image acquisition feature on Selenia Dimensions 3D systems," the company said in a statement. "The C-View software module is an additional, optional offering that, upon FDA approval, will provide an alternative solution to facilities wishing to address patient concerns about radiation exposure."
The FDA now takes the panel's recommendation under consideration, and it can approve or reject Hologic's submission. The company cannot predict when or if an FDA approval will be received, but Hologic said it plans to continue to work closely with the agency as it completes its final review of the C-View PMA.
The C-View module is currently commercially available for Selenia Dimensions 3D outside of the U.S., including countries in Europe, Latin America, and Asia, Hologic said.