
VIENNA - A flagship ultrasound scanner optimized for the coming age of artificial intelligence (AI), as well as a CT scanner designed to simplify dual-energy scanning, are among the new product introductions in the booth of GE Healthcare at ECR.
Ultrasound
In ultrasound, Logiq E10 will become GE's premium flagship ultrasound scanner, designed for radiology, shared services, and multiple other clinical applications outside of cardiology and ob/gyn scanning. This week's ECR 2018 meeting marks the system's global introduction, with a U.S. launch expected in the next several weeks.
 The Logiq E10 scanner was designed with powerful graphics processing unit chips inside.
The Logiq E10 scanner was designed with powerful graphics processing unit chips inside.Logiq E10 is based on GE's cSound Architecture platform, which acquires and reconstructs data in a similar manner to an MRI or CT system, allowing for 10 times the processing power of previous systems and 48 times the data throughput, according to GE. Logiq E10 also eliminates the need for focal zones because the entire image is always in focus throughout the exam; this provides for better image uniformity.
In designing the system, GE wanted to create a platform that would support the need to analyze and process massive volumes of data, as will be required in the coming age of artificial intelligence. Therefore, GE incorporated graphics processing units (GPUs) from NVIDIA into the scanner's architecture, giving the computer the horsepower to handle a variety of AI needs.
To improve image connectivity, the scanner supports GE's Photo Assistant App, which allows users to photograph relevant anatomy and include the photos with clinical images sent to the radiologist in DICOM format. Meanwhile, a tool called Remote Clinical Application enables radiologists to manipulate the ultrasound scanner's settings with a remote control on their tablet or smartphone. It is connected to the cloud via Trice Imaging's Tricefy, which adds cloud-based image sharing, remote viewing, and archiving and electronic health record (EHR) integration, the company said.
Women's imaging
In women's imaging, GE is demonstrating Senographe Pristina Dueta, a new system that allows women to control their own breast compression. Dueta was first shown at RSNA 2017.
As a work-in-progress at this year's meeting, GE is showing a new device that attaches to the company's Senographe Pristina mammography system that gives Pristina users more feedback on how to control their breast compression.
The device addresses the dilemma of how much compression to use with screening. For example, women with smaller breasts may not need the same amount of compression that women with larger breasts require. Without better guidance, technologists might apply too much compression, resulting in an exam that's too painful for women, or not enough compression, which could result in suboptimal image quality.
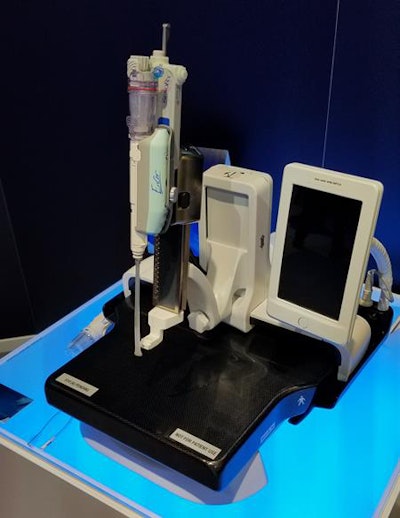 Pristina Serena is designed to enable interventional biopsy procedures to be performed on the Senographe Pristina mammography system.
Pristina Serena is designed to enable interventional biopsy procedures to be performed on the Senographe Pristina mammography system.The device consists of a special compression paddle embedded with sensors that measure the size of a woman's breast; it then calculates the resulting compression force that should be delivered to achieve optimal pressure. The second piece is a device with LED lights that illuminate as the optimal compression level is reached based on the woman's breast size, as well as whether the optimal level is exceeded.
GE sourced the device through an OEM agreement with Sigma Screening; the first installation will happen in March, with regular commercial deliveries scheduled for the second half of 2018.
Finally, GE is showing an interventional biopsy component for Senographe Pristina called Pristina Serena. Serena is a motorized biopsy needle holder that easily attaches to Pristina's bucky. Any vendor's needle can be used with the holder, which also can be rotated from a vertical to horizontal position based on what the provider determines to be the best approach for the suspicious lesion.
In Europe, Serena will begin shipping in production quantities in the second quarter. GE has submitted a 510(k) application for the product to the U.S. Food and Drug Administration.
CT
In its ECR booth, GE also is promoting Revolution Frontier, a CT scanner first launched at RSNA 2017. Frontier, a 128-slice scanner with a 0.35-sec gantry rotation speed, is designed to make it even easier to perform spectral CT imaging. Frontier features a new imaging chain, Gemstone Clarity detector, and Performix HD Plus x-ray tube; the combination of these technologies enables the scanner to achieve anatomic detail of 0.23 mm with 25% less electronic noise, the firm is telling ECR attendees.
Frontier includes GE's Gemstone Spectral Imaging (GSI) Pro, which delivers reconstruction speeds that are twice as fast as those of the previous GSI software for routine spectral workflow. It also supports the company's ASiR-V adaptive statistical iterative reconstruction algorithm for reducing dose; in fact, the scanner has real-time ASiR-V data processing for spectral scans, which should speed up workflow for dual-energy studies. Dual-energy scans processed with ASiR-V can now be sent directly to PACS, without the need for an additional processing step.
GE has an initial Revolution Frontier system installed at Saint-Joseph Hospital in Paris; commercial shipments are expected in June. The system already has U.S. Food and Drug Administration clearance and is awaiting the CE Mark.
MRI
In MRI, GE is highlighting advancements shown at the RSNA 2017 meeting, including Signa Premier, a new wide-bore 3-tesla scanner. The system sports a 70-cm short-bore architecture with GE's SuperG gradient coils, which are designed to provide the performance of a research-class 60-cm system in a 70-cm package. Signa Premier is also able to perform a brain exam in less than five minutes using the company's HyperSense scanning tool that delivers much faster scanning.


















