A deep-learning algorithm outperformed a top breast cancer risk assessment model in research presented at the virtual RSNA 2020 meeting. In fact, the algorithm bested the human risk assessment tool using data from mammograms alone.
The algorithm was created by a team from Harvard's Massachusetts General Hospital (MGH) and the Massachusetts Institute of Technology. The researchers trained, validated, and tested the algorithm using bilateral screening mammograms from more than 80,000 women.
The new algorithm achieved much higher accuracy than the Tyrer-Cuzick model, one of the most comprehensive breast cancer risk assessment tools available. However, its real strength was its ability to identify unique biomarkers from mammograms without the need for additional clinical risk factors, such as family history or age at first birth.
"Traditional risk models can be time-consuming to acquire and rely on inconsistent or missing data," stated Dr. Leslie Lamb, a breast radiologist at MGH and the lead authors of the research team, in a press release. "A deep learning image-only risk model can provide increased access to more accurate, less costly risk assessment and help deliver on the promise of personalized medicine."
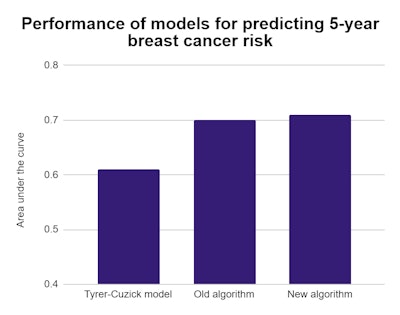
In a test with 9,290 mammograms, the Tyrer-Cuzick risk model achieved an area under the curve (AUC) of 0.61 for predicting five-year breast cancer risk. Meanwhile, the researcher's deep-learning algorithm netted an AUC of 0.71, a significant difference with a p-value of < 0.001.
The algorithm also outperformed a previous version that included both mammogram and clinical risk factors. The team theorized the newer algorithm is identifying biomarkers highly predictive of future cancer risk on mammograms that traditional risk models don't capture.
 Dr. Constance Lehman, PhD. Image courtesy of the RSNA.
Dr. Constance Lehman, PhD. Image courtesy of the RSNA."Every woman's mammogram is unique to her just like her thumbprint," stated Dr. Constance Lehman, PhD, the division chief of breast imaging at MGH and senior author of the research team. "It contains imaging biomarkers that are highly predictive of future cancer risk, but until we had the tools of deep learning, we were not able to extract this information to improve patient care."
The new research benefited from having a large population representative of many types of patients, including women with a personal history of breast cancer, who have implants, or who have had a biopsy. However, it was also limited to women who visited one of five screening sites that were part of a single academic institution, and 81% of the population was white.
The algorithm has since been externally validated in Sweden and Taiwan, and the team has plans to conduct additional studies in larger Black and minority populations in North America. With further development, the algorithm could help radiologists make full use of mammogram data, and radiologists at MGH already have access to deep-learning risk information on screening mammograms.
"Traditional risk assessment models do not leverage the level of detail that is contained within a mammogram," stated Lamb. "Even the best existing traditional risk models may separate sub-groups of patients but are not as precise on the individual level."