PET Myocardial Imaging:
Presently SPECT imaging is responsible for the great majority of cardiac imaging applications. Findings on the SPECT exam have both diagnostic and prognostic value. PET imaging has certain advantages includinghigher spatial resolution, improved attenuation correction, and the capabilityto perform quantitative measurements [17,37]. Like SPECT, PET myocardial imagingcan also provide diagnostic and prognostic information. In the detection of coronary artery disease, PET imaging has a sensitivity of 92%, a specificity of 89% (higher than SPECT--75%), and a normalcy of 89 to 95%. The higher specificity is related to the capability of PET imaging to provide attenuation correction that decreases the false positive rate. However, reconstruction artifacts producing false-positive defects can occur in up to 21% of cases due to mis-registration between transmission and emission scans [32]. Careful review of superimposed transmission and emission data sets should be performed to ensure correct registration [32]. Acquiring separate rest and stress transmission scans can help to reduce mis-registration artifacts [32].
At the present time, stress testing with PET must be performed with a pharmacologic agent [29]. Because the exercise component of a stress test in patients who are able to exercise provides independent prognostic information, the use of PET imaging should be reserved for patients unable to exercise, those with a prior equivocal SPECT exam, or in whom body habitus would degrade SPECT imaging [29].
PET Perfusion Imaging
Physiology of perfusion imaging
PET perfusion imaging
Anadvantage of PET perfusion imaging compared to SPECT imaging is that global andregional myocardial blood flow can be quantified in ml/gm/min. The benefits ofquantification are that the functional significance of coronary stenoses can be assessed directly, multivessel disease producing balanced ischemia can be detected, and the effects of medications or interventional cardiac procedures can be objectively measured.The ideal PET perfusion agent would be irreversibly trapped in the myocardium indirect relation to perfusion and have rapid blood clearance. The agents mostcommonly used for myocardial blood flow determinations are 13N-ammonia (13NH3), 15O labeled water (H215O), and 82Rubidium [29]. Because of poor extraction at high flow rates and relatively worse spatial resolution, 82Rubidium provides the least accurate estimate of myocardial blood flow [33]. 15O labeled water is theoretically superior to 13N-ammonia because it is a freely diffusible tracer with virtually complete myocardial extraction that is nearly independent of both flow rate and myocardial metabolic state [33]. When 15O labeled water is used, the myocardial blood flow (MBF) is estimated from the tracers washout from the myocardium, whereas for 13N-ammonia the myocardial tracer uptake is used to determine MBF [33].
The initial minutes of a dynamic
acquisition are the portion of the exam that is used for blood flow
quantification. Because of the limited count rate capabilities of PET scanners
and varying tracer kinetics, mathematic models must be applied for proper
quantification [29]. Methods have been developed to separate the delivery of tracer
to the heart (blood activity) from tracer retention in the myocardium.
Myocardial
activity is determined by a region of interest (ROI) placed over the myocardial
wall. Blood activity can be measured accurately by direct sampling of blood from
an artery, but this is invasive and the sample would still require correction
for time delays and dispersion. Measurements performed from the reconstructed
PET images would be more practical and less invasive.
The
most accurate site to measure arterial blood pool activity is at the level of
the coronary ostia at the base of the ascending aorta because this is the point
from which arterial blood irrigates the myocardial tissue. Unfortunately,
measuring activity from a ROI placed within the ascending aorta is subject to
error due to the limited resolution of the imaging system, the small dimensions
of the aorta, and contamination from adjacent vascular and myocardial
structures. Thus, activity is usually measured from an ROI placed within the
left atria or left ventricle. The drawback of a left ventricular ROI is that it
is subject to motion artifact as well as spillover activity from myocardial
tissue. A left atrial ROI tends to closely match values obtained from direct
arterial sampling, however, it is often more difficult to identify the left
atrial chamber on PET images. When using a left atrial ROI it is important to
correct for the small time delay between the arrival of the blood in the left
atria and its actual arrival in the myocardium or flow rates may be
artificially elevated.
Previously, attenuation correction of myocardial imaging was performed with a 68Ge transmission source [31]. Newer PET/CT scanners use CT for attenuation correction and this has also been shown to provide accurate results for qualification and quantification of myocardial perfusion [31]. PET/multi-detector CT permits the fusion of anatomic and functional information and can accurately measure atherosclerotic burden and identify flow-limiting coronary stenoses [37].
Radiopharmaceuticals/Technique for perfusion imaging
Agents used for PET myocardial perfusion imaging include 13N-ammonia, 15O-water, Rubidium-82, and 62Cu -PTSM. Agents labeled with 18F (such as 18F-fluorodihydrorotenone) are being developed for clinical use [36].
13N is cyclotron produced by either irradiating methane gas with deuterons [12C (d,n) 13N] or by the reaction 16O (p,alpha) 13N with water as the target. 13N has a physical half-life of 10 minutes. There is rapid blood clearance with high initial extraction (over 90%) and high tissue retention (80%), which provides high myocardial to background count ratios. Tracer uptake in the lungs in smokers and in patients with congestive heart failure can interfere with imaging [29]. Liver uptake can also be problematic [29].
The agent diffuses into myocytes where it is metabolized to 13N-labeled glutamine and becomes unable to leave the cell. Extraction of the tracer generally reflects regional perfusion, however, extraction decreases at high flow states (to as low as 35%) and plateaus at flow rates greater than 2 mL/gm [29]. Another consistent finding is low uptake of the tracer in the lateral/posterolateral wall on both stress and rest images which appears to be related to a regional alteration in 13N tissue metabolism/retention [3]. This defect is not detected on dynamic imaging, which can be used to differentiate a true perfusion defect from artifact [4].
The critical organ for N-13 is the urinary bladder. The typical dose used for imaging is 10 to 15 mCi I.V. given over a 20 to 30-second interval. Imaging is usually started 5 minutes after injection of the tracer to permit pulmonary background activity to clear. Images should be acquired in a decay compensated mode. A FDG exam may be performed after a 50-minute interval to permit decay of the N-13 to background levels.
15O is cyclotron produced by the irradiation of nitrogen [14N (d,n) 15O or 15N(p,n)15O] and it has a physical half-life of only 2.2 minutes (123 seconds). O-15 produced in the cyclotron is converted to [15O]CO2 by passing it over activated charcoal at 400 to 600 degrees Celsius. [O-15] water is then prepared by bubbling the [15O]CO2 into water. Theoretically, 15O water is ideal for quantitative regional myocardial flow measurements (in ml/min/gm) for two reasons: it is a freely diffusible perfusion tracer with 100% extraction by the myocardium [29], and it is not affected by metabolic factors.
The extraction of oxygen-15-water (H215O) remains linear even at very high flow rates (it is the only perfusion tracer with linear extraction) and, therefore, myocardial distribution of the agent reflects regional perfusion. Unfortunately, image quality is not as good as that obtained with other flow agents because tracer circulating in the blood pool remains within the ventricular chamber and must be subtracted in order to visualize the myocardium. This can be done by use of a separate administration of O-15 carbon monoxide to label erythrocytes or by the use of an early image (20-40 seconds after the administration of O-15 water) which would represent a vascular image before significant activity reaches the coronary arteries [29].
For myocardial perfusion quantification, dynamic scans are obtained for up to 5 minutes after bolus administration of 15-25 mCi [29].
Rubidium-82 (82Rb) is a generator produced positron emitter. The agent is produced from Strontium-82 (T1/2=25.5 days) which decays by electron capture to 82Rb. Rubidium-82 is a potassium analog with a physical half-life of only 75 seconds. The short half-life allows for repeated blood flow measurements in short time intervals. One drawback of the agent is that myocardial extraction of the agent is low- about 50-60% at rest and this further decreases at high flow rates to only 25-30%. Myocardial ischemia and reperfusion reduce the uptake of Rb-82 due to a reduction in cellular transport [29]. This reduction in extraction occurs even after only short periods of transient ischemia [29]. The short half-life of the agent also has some negative effects as well. Following injection imaging must be delayed for at least 2 minutes to permit clearance of blood pool activity and considerable decay of the tracer occurs during this time. Also, due to changing myocardial activity from decay, imaging times must be short to prevent back-projection reconstruction artifacts.
|
Rb-82 rest perfusion examination |
|
|
Exam protocol: Rest imaging should be performed prior to stress imaging to reduce the impact of residual stress effects such as myocardial stunning which can affect Rb-82 extraction [27,29]. The typical dose for the 82Rb perfusion exam is 40 to 50 mCi infused over 20 to 30 seconds [27]. In patients with normal LV function, imaging is usually begun 60-70 (up to 90) seconds after the infusion is complete to permit clearance of blood pool activity. Imaging is delayed to 90-110 seconds following completion of the infusion in patients with poor LV function (LVEF less than 30%) [27]. About 80% of the useful counts are acquired in the first 3 minutes, and 95% in the first 5 minutes [27]. 82Rb has the worst resolution of all the positron emitting agents, with most positrons traveling about 12.4 mm prior to undergoing annihilation. The critical organ is the kidney.
For a standard 2D acquisition 82Rb cardiac images tend to be count poor due to the agents short half-life [26]. 3D imaging has a higher sensitivity, however, it suffers from greater scatter, random events, and saturation which can paralyze a system [26]. Further work needs to be performed to determine optimal 3D myocardial perfusion imaging [26].
Copper 62 PTSM: Copper-62-Pyruvaldehyde-bis-(4N-thiosemicarbazone)
Cu-PTSM is a generator-produced tracer which can also be used for myocardial perfusion imaging. 62Cu has a physical half-life of 9.7 minutes. The short half-life of the parent isotope 62Zn (9.3 hours) limits the practical life of the generator to 1 to 2 days. However, once loaded, the generator can be eluted every 30 minutes.
62Cu -PTSM is a neutral, lipophilic tracer that is taken up in tissues and then trapped intracellularly by reduction to a non-lipophilic compound. There is non-linear extraction of the tracer (ie: extraction decreases at high flow rates) and high liver activity which scatters into the inferior wall. The agent also binds to human albumin which precludes accurate recording of the arterial input function which is critical for quantification. [5]
PET Metabolic Imaging
There are numerous clinical applications for PET metabolic myocardial imaging. One of the most recognized applications is for the assessment of viable myocardial tissue. As with perfusion, myocardial metabolic functions can also be quantified by PET imaging.
Physiology of metabolic myocardial imaging:
The heart can oxidatively metabolize a variety of substrates to meet its high energy needs. In the fasting state (high circulating free fatty acids and low insulin levels) the myocardium preferentially utilizes fatty acids and lactate as aerobic substrates to provide the energy (ATP) necessary for contractile function (unlike other cells in which glucose is the most important substrate). In plasma, fatty acids are transported bound to albumin and enter the myocardial cell either by passive or active transport. Once in the cell, fatty acids undergo oxidative metabolism. Although fatty acids are the preferred myocardial energy substrate, even in the fasting state glucose utilization is variable and can still account for 30-40% of the energy derived from oxidative metabolism [15].
Fatty acid beta-oxidation occurs in the mitochondria and is very sensitive to oxygen deprivation. When blood flow is significantly reduced, tissue delivery of oxygen and removal of waste products are also reduced. As a result, oxygen dependent substrate catabolism decreases. Since fatty acids are catabolized by beta oxidation, fatty acid consumption ceases with anoxia (i.e.: a depression of fatty-acid extraction and oxidation will occur in zones of myocardial ischemia or infarction). This decrease in fatty acid utilization is followed by an increased glycolytic flux with glycogen depletion and increased exogenous glucose utilization.
During periods of ischemia or hypoxia the myocyte compensates for the loss of oxidative potential by shifting toward glucose utilization to generate high-energy phosphates. Unfortunately, the amount of energy produced via glycolysis may not be sufficient to sustain mechanical work, but it can be adequate to maintain cell viability--this condition is referred to as hibernating myocardium. Glycolysis can only be maintained if lactate and hydrogen ions (the byproducts of glycolysis) do not accumulate intracellularly. Therefore, blood flow must be sufficient to deliver glucose to the cell and remove the metabolites of the glycolytic pathway. Once perfusion is decreased below a critical level, the tissue concentrations of lactate and hydrogen ions will increase and inhibit glycolysis [14]. This results in a loss of ion concentration gradients across the cell membrane, followed by cell membrane disruption, and cell death [14].
PET Metabolic Myocardial Imaging using 18F-2-Deoxyglucose (18-FDG) and exam technique:
18F-2-deoxyglucose is one of the agents used for cardiac metabolic imaging. Fluorine-18 has an effective half life of 110 minutes. It is produced by bombarding 18O with protons and displacing a neutron [18O (p,n) 18F]. Chemically 18F is comparable in size to hydrogen. 18F has the best resolution of all positron emitters--approaching 2 mm. This is because most of the emitted positrons traveling only about 1.2 mm prior to undergoing an annihilation reaction.
The agent 18FDG competes with glucose for facilitated transport into myocardial cells and once inside the cell it competes for phosphorylation by hexokinase. Unlike glucose, however, the phosphorylated form is not further metabolized. Therefore, regional myocardial uptake of 18FDG reflects regional rates of exogenous glucose utilization. Only about 1 to 4% of the injected dose is trapped in the myocardium, but the target to background ratios are favorable (Heart:Lung [20:1], Heart:Blood [14:1]).
|
.Myocardial PET FDG imaging: |
|
|
18FDG uptake in the myocardium is highly dependent on the patient's dietary state [14]. Myocardial glucose utilization is increased by glucose administration (ie: such as following a meal) which stimulates insulin secretion. The increased insulin levels stimulate glucose metabolism, and tissue lipolysis is inhibited [15]. Therefore, in normal myocardium, 18FDG, uptake will be promoted in association with high glucose/insulin levels, but decreased in the face of high free-fatty acid levels (ie: with fasting). Unlike normal myocardium, regions of severely decreased perfusion (hibernating myocardium) preferentially utilize glucose as an energy substrate during periods of fasting in order to maintain cell viability. It is this difference in substrate utilization between normal and hibernating myocardium that forms the basis for PET myocardial viability imaging.
For metabolic imaging, patients fast for at least 6 hours and then receive a glucose load [27]. Several protocols are available to promote cardiac FDG uptake:
- Oral glucose loading
- Hyperinsulinemic euglycemic clamping
- Administration of nicotinic acid derivatives
In patients with diabetes the increase in plasma insulin levels following glucose loading may be attenuated and the cells may be less able to respond to insulin stimulation [15,27]. Consequently, tissue lipolysis is not inhibited, plasma fatty acid levels remain high, and myocardial glucose uptake can be poor [15]. This can result in suboptimal quality exams. An improvement in image quality can be obtained by waiting 2-3 hours after tracer injection before imaging (at the expense of increased FDG decay) [27]. In diabetic patients following glucose loading, an IV bolus of regular insulin is given according to a sliding scale and the plasma glucose is checked every 15 minutes in order to maintain a stable serum glucose level of approximately 140 mg/dL.
FDG imaging in patients with non-insulin dependent diabetes is also problematic [20]. An oral glucose load and insulin boluses can be used to control blood glucose levels [20]. For a fasting plasma glucose level less than 7 mmol/L, a 25 g oral glucose solution is given; for 7-11 mmol/L , 5 IU of insulin is given I.V.; for a fasting glucose greater than 11 mmol/L, 10 IU of insulin is given I.V. [20]. The plasma glucose level is then measured in 15 minutes and insulin given according to a sliding scale: For 8 mmol/L no insulin is given; for 8-11 mmol/L, 5 IU of insulin is given I.V., and for a glucose level greater than 11 mmol/L, 10 IU of insulin is given I.V. [20].
Hyperinsulinemic-euglycemic clamping: Hyperinsulinemic-euglycemic clamping requires the simultaneous infusion of glucose and insulin to achieve perfect metabolic regulation [22]. With the use of the clamp, high insulin levels, stable glucose levels, and low free fatty acid levels can be obtained [22]. The hyperinsulinemic-euglycemic clamp method has been shown to produce images with the highest myocardial to background ratio [20] even in diabetic patients, however, the method is time consuming and not practical for clinical work [15]. The procedure requires IV's placed in both the left and right arms- one is used for insulin infusion and the other for glucose. Insulin is infused at a rate of 100mU/kg/hr to achieve hyperinsulinemia [22]. A simultaneous glucose infusion (500mL of 20% glucose with 20mL of 14.9% potassium chloride to prevent hypokalemia) is infused at a rate ot 6 mg/kg/min and the rate is adjusted every 10 minutes to maintain normoglycemia [22]. After 60 minutes of clamping, the FDG is administered intravenously [22].
Nicotinic acid derivatives: Nicotinic acid derivatives have also been studied for use with PET FDG myocardial imaging [22]. The agent acipimox inhibits peripheral lipolysis and thereby reduces circulating free fatty acids [22]. Acipimox 250 mg is orally given 2 hours prior to FDG injection. Some centers will also give patients a carbohydrate and protein-enriched meal [22]. Image quality is generally very good (even in patients with diabetes [25]), with uninterpretable exams in about 5% of patients [22]. The effectiveness of this agent in patients with diabetes requires further study [22].
The typical dose used for the exam is 15 mCi infused over a 60 second interval. Images are usually acquired 45 minutes following administration of the radiotracer [30]. When performing attenuation correction with the use of a transmission scan, ideally the transmission scan should be performed prior to FDG injection. Post-injection transmission scans may suffer from emission contamination and the resultant attenuation corrected images can underestimation FDG activity by about 20% unless an emission spillover adjustment is applied [30].
PET FDG imaging for the detection of viable myocardial tissue
Hibernating myocardium represents severely ischemic, but viable myocardial tissue. In this condition there is a chronic reduction in myocardial metabolism and contractility in order to match a long-standing decrease in blood supply (chronic ischemia). In other words, the cells maintain viability, but energy production is insufficient to maintain mechanical work and contractile dysfunction results [1]. This may be a protective response by the myocytes to reduce their oxygen demand in the setting of reduced oxygen availability. This is a reversible phenomenon and ventricular contractile dysfunction can improve if flow is restored. Because enhanced left ventricular function after revascularization is associated with improved survival, it is crucial to identify areas of viable myocardial tissue.
Evaluation of glucose metabolism using 18FDG remains the gold standard for the evaluation of myocardial viability (hibernating myocardium) [1,6,19]. Ischemic, but viable myocardium (hibernating) utilizes glucose in preference to other substrates. Ischemic myocardium will therefore concentrate an increased amount of 18FDG compared to normal myocardium. Increased utilization of glucose can also be seen in myocardium recovering from a recent ischemic event as enhanced anaerobic glycolytic activity persists for some time after reperfusion, despite the availability of adequate oxygen.
The viability examination is usually performed following some form of glucose loading even though this seems to be contradictory. It would seem most logical to perform 18FDG myocardial viability imaging in the fasting state when normal myocardial glucose utilization is very low (normal myocardium uses fatty acids for energy substrate in the fasting state). Unfortunately, fasting 18FDG imaging of the myocardium may be overly sensitive -- detecting small, clinically insignificant areas of myocardial viability because of low background myocardial activity. Additionally, the normal myocardium can be difficult to define in the fasting state and the tracer may not clear adequately from the blood, which can result in image degradation. The one drawback of glucose loading exams is that myocardial substrate utilization will shift from fatty acids to glucose and small areas of viable myocardium may be missed. In either case, the average positive predictive accuracy for detecting viable myocardium by fasting FDG studies is about 85% and is similar to post-glucose loading exams (81%).
Severely decreased FDG uptake below 50% of normal myocardial activity is generally considered indicative of scar, while uptake greater than 50% of normal myocardial activity is indicative of viability. However, determination of ischemic, but viable myocardium can only be made in relationship to a perfusion study [7]. The perfusion exam does not need to be performed with a PET agent; it can be performed using SPECT tracers such as thallium or technetium agents [18]. When regional myocardial 18FDG uptake is disproportionately enhanced when compared with regional myocardial blood flow, the pattern is termed a perfusion-metabolism mismatch. In the setting of chronic coronary artery disease, a perfusion-metabolic mismatch is highly predictive of myocardial viability and indicates a high likelihood of improved cardiac function following revascularization (improved contractility is seen in 80%-85% of cases and the average improvement in ejection fraction (EF) is 15%).
A scar is characterized by concordant reduction in perfusion and FDG uptake (a FDG-perfusion match) [14]. Improved wall motion is seen in only 8-17% of matched perfusion-metabolic defects [14]. Based on the severity of the perfusion and FDG deficit, the matched pattern may be categorized as a transmural match (absent or markedly reduced perfusion and FDG uptake) or a non-transmural match (mild to moderately reduced perfusion and FDG uptake). When tracer activity equals 50 to 60% or more of peak activity, the matches are mild and probably represent a non-transmural scar [14]. Contractile reserve is more likely to be seen in regions of non-transmural MI [23]. If quantification is performed, viable myocardium is very unlikely to exist if the basal myocardial blood flow is less than 25 ml/min/100 gm [8].
Although the uptake of 18FDG can discriminate between viable and non-viable tissue, the regional myocardial blood flow and 18FDG uptake pattern is similar for varying types of myocardial dysfunction. Clinical symptoms are necessary to determine the exact etiology of the scan findings. For instance, increased utilization of glucose can also be seen in myocardium recovering from a recent ischemic event as enhanced anaerobic glycolytic activity persists for some time after reperfusion, despite the availability of adequate oxygen. Different conditions may also coexist in the same myocardial regions.
Condition |
rMBF |
FDG Uptake |
Clinical State |
| Acute ischemia | Decreased | Normal or increased | Chest pain/Angina |
| Hibernating | Decreased | Normal or increased | Chronic stable |
| Stunning | Normal | Normal or increased | Following acute event |
| Necrosis/Scar | Decreased | Decreased | Chronic symptoms |
Prognostic Implications of FDG PET Myocardial Imaging (General):
Cardiac events and mortality:
PET studies have shown that cardiac morbidity and mortality is increased in patients with flow-metabolic mismatches [18]. Up to 50% of patients that demonstrate a perfusion-metabolic mismatch will have a cardiac event in the subsequent 12 months in the absence of intervention. The greater the number of mis-matched segments identified, the greater the risk for subsequent cardiac event if revascularization is not performed [18]. The incidence of cardiac events drops to 15% in these patients if revascularization is performed [9]. In two other studies, mortality ranged between 4 to 12% in the group with matched defects, and between 33 and 41% in the mismatch group. In the mismatch group, if revascularization was performed, mortality dropped to between 4 and 12% [7].
In patients with underlying left ventricular dysfunction:
It is well recognized that in patients with coronary artery disease (CAD) on medical therapy, the presence of left ventircular (LV) dysfunction is associated with a high mortality. In the Coronary Artery Surgery Study (CASS), mortality of medically treated patients was related to the severity of LV dysfunction with up to 25% annual mortality in patients with resting LVEF's below 25%. In patients with LVEF's below 35% survival was better in those patients undergoing revascularization. Unfortunately, revascularization cannot be recommended in all patients with poor LV function because the surgery is associated with a 5 to 35% mortality in this subgroup. Ideally, the surgery should be performed only in those patients with a high likelihood of improved LV function.
In patients with symptoms of cardiac failure a PET pattern of perfusion-metabolic mismatch identifies a subgroup of patients who are at very high risk for cardiac death on medical therapy (30-40%). These patients are most likely to show significant clinical improvement and have prolonged survival as a result of revascularization [1,18].
For the prediction of improvement in regional LV function after revascularization, 18FDG -PET imaging has a pooled sensitivity of 88%, a specificity of 73%, a positive predictive value of 76%, a negative predictive value of 86%-92% [14, 19, 21], and an accuracy of 68%-95% [16]. As a general rule, the larger the extent of the perfusion-metabolic mismatch, the greater will be the anticipated improvement in LVEF following revascularization [34]. If 17% [37] to 25% or more of the LV is viable, a significant improvement in LVEF can be expected following revascularization [14]. Patients with large mismatches also achieve significantly higher functional status compared to patients with minimal or no PET mismatch [34]. A matched perfusion-metabolism defect is 75%-100% accurate in predicting that segmental wall motion will not improve [16]. Improved wall motion is seen in only 8%-17% of matched perfusion-metabolic defects [14]. Unfortunately, not all patients with evidence of viable myocardium on PET imaging demonstrate improvement in wall motion following revascularization [24]. Despite a substantial amount of viable myocardium these patients may have endocardial necrosis (the endocardium contributes almost 75% to total wall thickening at rest) [24] or severe ultrastructure damage which limits contractile reserve [34].
The perfusion-metabolism mismatch pattern is also predictive of improvement in heart failure symptoms after revascularization [10]. In patients with heart failure symptoms and a PET perfusion-metabolic mismatch, 70% had improvement in symptoms following revascularization, while only 30% without a mismatch experienced improvement. Improvement in heart failure symptoms correlates with the preoperative extent of viable myocardium [37]. Patients with a PET mismatch pattern involving 18% or more of the LV volume appear to show the greatest clinical benefit [37]. In patients with ischemic cardiomyopathies being considered for revascularization, LVEF and LV volume information determined from gated FDG PET provide incremental prognostic value [35]. Patients with LVEF's below 25% or EDV's greater than 260 mL may not demonstrate improvement in heart failure symptoms following revascularization (although they may still derive a survival benefit) [35]. This is most likely related to advanced ventricular remodeling [35].
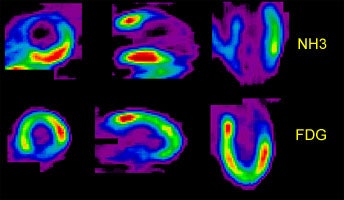
Perfusion-Metabolism Mismatch: The case below is from a 62-year-old female patient 8-years status post CABG and 6 months following an anterior wall myocardial infarction. The patient presented with symptoms of congestive heart failure and a LV ejection fraction of 22%. Perfusion exam was performed using NH3. There is an extensive, severe perfusion defect involving the apex, anterior, and septal walls. Metabolic images performed using FDG demonstrate disproportionately enhanced uptake when compared to the perfusion study. This is referred to as a perfusion-metabolism mismatch which is considered reflective of hibernating myocardium. At angiography the patient was found to have an occluded bypass graft and a second bypass was performed. Six months later the patient was asymptomatic and the LV ejection fraction had increased to 47%. Case courtesy of CTI, Inc. |
|
In the Immediate Post Infarct Period:
In the immediate post infarction period (out to 1-week post event) the functional outcome of infarcted segments with a flow-metabolism mismatch pattern is more variable and does not necessarily imply recovery of contractile function following revascularization (ie: in the post infarct period PET may overestimate the potential for recovery) [8].
Limitations of PET metabolic imaging:
Limitations of FDG imaging include limited data in diabetic patients and recent reports that suggest that increased FDG accumulation can be seen in acute evolving myocardial infarctions [1, 11]. In the immediate post infarction period (out to 1-week post event) the functional outcome of infarcted segments with a flow-metabolism mismatch pattern is more variable and does not necessarily imply recovery of contractile function following revascularization (for example, in the post infarct period PET may overestimate the potential for recovery) [8]. Also, in the evaluation of FDG-PET for myocardial viability there is wide variability in the literature regarding patient inclusion criteria and examination protocol [14].
Other Approaches to Metabolic Myocardial Imaging
In addition to standard perfusion-metabolic imaging using 18FDG, other approaches have also been utilized to assess for viability, including determination of oxidative metabolism with 11C palmitate, uptake and retention of 82Rb, 18F fluoroisonidazole imaging, and the water-perfusable tissue index.
PET Metabolic Myocardial Fatty Acid Imaging
C-11 palmitate
Long chain fatty acids account for 90% of the myocardial energy requirements in the fasting state. When glucose or insulin levels are high, such as after a meal, glucose oxidation increases and fatty acid use is suppressed [2]. During ischemia, glucose again plays a major role in oxidative metabolism, whereas oxidation of long chain fatty acids is greatly reduced [2].
Long-chain free fatty acids easily pass through the sarcolemmic membrane to be activated as acylcoenzyme A (CoA). Acyl CoA is carried into mitochondria through an acyl carnitine carrier system to enter the beta-oxidation pathway. After beta-oxydation , acetyl CoA is formed and enters into the tricarbonic acid cycle for further oxidation to become water and carbon dioxide. Palmitic acid comprises approximately 25 to 30% of the circulating fatty acid in the blood and serves as one of the primary sources for energy production by the heart. The distribution of C-11 labeled palmitic acid reflects efficiency of myocardial energy production.
After initial uptake of the tracer, there is a rapid clearance component which corresponds to levels of beta-oxidation. This is followed by a slower clearance component which reflects incorporation of the tracer into triacylglycerols and phospholipids.
In normal myocardium, there is homogeneous distribution of the tracer. During periods of ischemia, the uptake of the agent and the rate of the rapid clearance phase and its magnitude markedly diminish, consistent with diminished oxidative metabolism. Delayed clearance is also observed from ischemic and infarcted regions as a result of decreased fatty acid oxidation. C-11 palmitate exams reveal segmental reductions of blood flow in acutely ischemic myocardium are associated with delayed clearance of 11C-palmitate and an increase in 18-FDG uptake.
The rapid blood clearance of this agent and loss of radioactivity from myocardial tissue due to straight-chain fatty-acid catabolism by beta oxidation complicate data analysis. A drawback of this agent is that it is affected by plasma substrate concentrations--in the fasting state, when glucose levels are low, fatty-acid metabolism is accelerated and C-11 palmitate washout is rapid. Washout decreases markedly with glucose loading. The critical organ is the liver.
1-[11C] acetate
Another agent, 1-[11C] acetate can also be used to assess overall oxidative metabolism. The tracer is well extracted by the myocardium and is then converted to acetyl-CoA. Acetyl-CoA is the entry point for all metabolic pathways into the tricarboxylic acid cycle within the mitochondria. Oxidation of acetyl-CoA leads to the production of C-11 labeled CO2 reflecting the activity of the tricarboxylic acid cycle that, under normal conditions, reflects overall oxidative metabolism.
Even in the face of changing patterns of myocardial substrate use, the clearance of 1-[11C] acetate accurately delineated overall oxidative metabolism (ie: unlike C-11 palmitate, this agent is not affected by plasma substrate concentrations). The maintenance of oxidative metabolism in jeopardized myocardium was identified as a prerequisite to recovery of function following revascularization. Myocardial uptake and clearance are homogeneous in normal subjects. In infarcted myocardium, there is decreased uptake and clearance of the tracer due to the reduced oxygen consumption.
The predictive accuracy of this tracer in viability assessment may be superior to FDG. In one study, 15% of nonviable myocardial regions demonstrated increased uptake of FDG relative to flow, but diminished levels of oxidative metabolism, while 20% of viable segments (demonstrating normal oxidative metabolism) had reduced FDG accumulation [11].
Rubidium-82
For Rb-82 imaging in the evaluation of myocardial viability there is unfortunately no association between the severity of a fixed defect on 82Rb imaging and the likelihood of myocardial viability in that area. In defects containing less than 50% relative activity, 30% were shown to be viable by FDG imaging [13].
18F-Fluoromisonidazole
18F-Fluoromisonidazole has been shown to have increased retention in hypoxic, non-infarcted myocardium. It can therefore be useful for the selective delineation of hypoxic, but viable tissue.
H2150 Water
(H215O) can be used to determine the water-perfusable tissue index. Contractile function is thought to improve after revascularization only if the perfusible tissue fraction is at least 70%.
REFERENCES:
(1) Radiographics 1999; Jadvar H, et al. SPECT and PET in the
evaluation of coronary artery disease. 19: 915-926
(2) J Nucl Med 2000; Tamaki N, et al. The role of fatty acids in cardiac imaging. 41: 1525-1534
(3) J Nucl Med 1994; Schwaiger M. Myocardial perfusion imaging with PET. 35: 693-98
(4) J Nucl Med 1995; de Jong RM, et al. Posterolateral defect of the normal human heart investigated with nitrogen-13-ammonia and dynamic PET. 36: 581-585
(5) J Nucl Med 1998; Wallhaus TR, et al. Human biodistribution and dosimetry of the PET perfusion agent Copper-62-PTSM. 39: 1958-1964
(6) J Nucl Med 1995; Delbeke D, et al. Rest myocardial perfusion/metabolism imaging using simultaneous dual-isotope acquisition SPECT with technetium-99m-MIBI/fluorine-18-FDG. 36: 2110-19
(7) J Nucl Med 1994; Schelbert HR. Metabolic imaging to assess myocardial viability. 35(4 Suppl):8S-14S. Review
(8) J Nucl Med 1995; Grandin C, et al. Delineation of myocardial viability with PET. 36(9):1543-52
(9) J Am Coll Cardiol 1992; Eitzman D, et al. Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. 20: 559-65
(10) J Nucl Med 1994; Maddahi J. Cardiovascular nuclear medicine: state-of-the-art. 35(4):672-3 (No abstract available)
(11) J Nucl Med 1994; Bergmann SR. Use and limitations of metabolic tracers labeled with positron-emitting radionuclides in the identification of viable myocardium. 35(4 Suppl):15S-22S. Review
(13) Radiology 1995; Go RT, et al. Hibernating myocardium versus scar: severity of irreversible decreased myocardial perfusion in prediction of tissue viability. 194:151-55
(14) Semin Nucl Med 2000; Bax JJ, et al. 18-Fluorodeoxyglucose imaging with positron emission tomography and single photon emission computed tomography: Cardiac applications. 30: 281-298
(15) J Nucl Cardiol 2001; Dilsizian V, et al. Fluorine-18-deoxyglucose SPECT and coincidence imaging for myocardial viability: Clinical and technical issues. 8: 75-88 (No abstract available)
(16) J Nucl Med 1993; Schelbert H, et al. Positron statement: Clinical use of cardiac positron emission tomography. Position paper of the cardiovascular council of the society of nuclear medicine. 34: 1385-88 (No abstract available)
(17) J Nucl Med 1993; Garcia EV, et al. What should we expect from cardiac PET? 34: 978-980 (No abstract available)
(18) J Nucl Med 2001; Zhang X, et al. Clinical outcome of patients with previous myocardial infarction and left ventricular dysfunction assessed with myocardial 99mTc-MIBI SPECT and 18F-FDG PET. 42: 1166-1173
(19) J Nucl Med 1998; Bax JJ, et al. Comparison of fluorine-18-FDG with rest-redistribution thallium-201 SPECT to delineate viable myocardium and predict functional recovery after revascularization. 39: 1481-1486 (No abstract available)
(20) J Nucl Med 2001; Vitale GD, et al. Myocardial glucose utilization and optimization of 18F-FDG imaging in patients with non-insulin-dependent diabetes mellitus, coronary artery disease, and left ventricular dysfunction. 42: 1730-1736
(21) J Am Coll Cardiol 1997 Bax JJ, et al. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: Pooled comparison data. 30: 1451-60
(22) J Nucl Cardiol 2002; Bax JJ, et al. Safety and feasibility of cardiac FDG SPECT following oral administration of Acipimox, a nicotinic acid derivative: comparison of image quality with hyperinsulinemic euglycemic clamping in nondiabetic patients. 9: 587-593
(23) J Nucl Med 2003; Schinkel AFL, et al. Dobutamine-induced contractile reserve in stunned, hibernating, and scarred myocardium in patients with ischemic cardiomyopathy. 44: 127-133
(24) J Nucl Cardiol 2003; Kaul S. What lies beyond a chronically occluded coronary artery, and what should we do about it. 10: 92-94 (No abstract available)
(25) J Nucl Med 2003; Schinkel AFL, et al. Effect of diabetes mellitus on myocardial 18F-FDG SPECT using acipimox for the assessment of myocardial viability. 44: 877-883
(26) J Nucl Med 2003; Knesaurek K, et al. Comparison of 2-dimensional and 3-dimensional 82Rb myocardial perfusion PET imaging. 44: 1350-1356
(27) J Nucl Cardiol 2003; Bacharach SL, et al. PET myocardial glucose metabolism and perfusion imaging: part I- guidelines for patient preparation and data acquisition. 10: 545-556 (No abstract available)
(28) J Nucl Cardiol 2003; Schelbert HR, et al. PET myocardial perfusion and glucose metabolism imaging: part 2- guidelines for interpretation and reporting. 10: 557-571 (No abstract available)
(29) J Nucl Cardiol 2004; Beller GA, Bergmann SR. Myocardial perfusion imaging agents: SPECT and PET. 11: 71-86 (No abstract available)
(30) J Nucl Med 2004; van der Weerdt AP, et al. Postinjection transmission scanning in myocardial 18F-FDG studies using both filtered backprojection and iterative reconstruction. 45: 169-175
(31) J Nucl Med 2004; Koepfli P, et al. CT attenuation correction for myocardial perfusion quantification using a PET/CT hybrid scanner. 45: 537-542
(32) J Nucl Med 2004; Loghin C, et al. Common artifacts in PET myocardial perfusion images due to attenuation-emission mis-registration: clinical significance, causes, and solutions. 45: 1029-1039
(33) J Nucl Cardiol 2004; Rimoldi OE, et al. Positron emission tomography for quantification of myocardial perfusion. 11: 482-489
(34) J Nucl Cardiol 2004; Maddahi J. Factors influencing predictive value of FDG imaging for evaluating myocardial viability. 11: 526-526
(35) J Nucl Cardiol 2004; Santana CA, et al. Incremental prognostic value of left ventricular function by myocardial ECG-gated FDG PET imaging in patients with ischemic cardiomyopathy. 11: 542-550
(36) J Nucl Med 2004; Marshall RC, et al. Kinetic analysis of 18F-fluorodihydrorotenone as a deposited myocardial blood flow tracer: comparison to 201Tl. 45: 1950-1959
(37) J Nucl Cardiol 2004; Di Carli MF. Advances in positron emission tomography. 11: 719-732