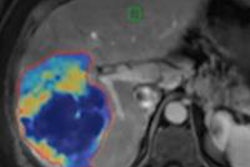
A semiautomated lesion management application within PACS software can provide reliable, consistent, and speedy segmentation of metastatic lesions in serial CT examinations of cancer patients, according to research published in the September issue of the American Journal of Roentgenology.
Not only did the software provide more consistent measurements than a manual method, it performed the tasks in about half the time, and it yielded more complete information in a digital form, according to a U.S. National Institutes of Health (NIH) team led by Dr. Les Folio.
"These results will be important for improved metastatic tumor burden assessment in cancer treatment [and] for clinical research trials, while likely becoming more widespread with ease of application in existing workflows," Folio told AuntMinnie.com.
RECIST criteria
Currently, cancer treatment response is evaluated by manual assessment of target lesions using the Response Evaluation Criteria in Solid Tumors (RECIST), which themselves are based on a percentage change in target lesions from baseline to follow-up images. It's challenging, however, to obtain timely, accurate, and consistent target measurements, and manual protocols also suffer from a number of other limitations, according to the NIH team (AJR, September 2013, Vol. 201:3 pp. 618-625).
In an attempt to address these shortcomings, the researchers sought to assess a semiautomatic lesion management application within their PACS software (Vue PACS, v11.4, Carestream Health) that is designed to facilitate detection, measurement, and data capture of target lesions.
In lung and liver lesions, the lesion management application assists with measurement recording, RECIST calculation, data input, and lesion segmentation. While lymph node segmentation was not available at the time of the NIH study, the application's features also assist in measurement recording, RECIST calculation, and data input for lymph node lesions, according to the authors.
Folio and colleagues assessed interobserver agreement for the manual method and the lesion management application, and they also calculated time savings over the conventional manual method.
Retrospective review
Two observers retrospectively and independently reviewed 93 target lesions (17 lung, five liver, and 71 lymph node lesions) in 50 patients who had either metastatic bladder or prostate cancer. The observers, who were postbaccalaureate students without medical training, measured the longest axis of the lesion (or short axis in the case of lymph nodes) and made RECIST determinations using a manual protocol and via the lesion management application. A radiologist with more than 15 years of body CT experience verified all measurements and segmentations after they were completed.
| Median time savings from lesion management software by scan type | ||
| Scan type | Observer 1 | Observer 2 |
| Follow-up scans | 45% | 28% |
| Baseline scan | 28% | 9% |
Analysis of the interreader variability of measurements showed that the percentage difference between the two readers in their lesion measurements was 8.9% when using the software and 26.4% without it.
"Segmentation of most lung and liver lesions with a semiautomated lesion management application method was more consistent between observers than was use of a manual method for all lesions and was significantly faster," the authors concluded.
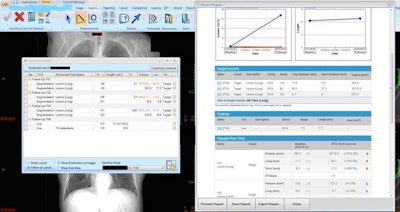
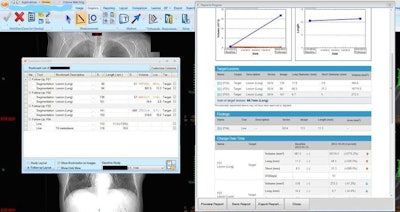
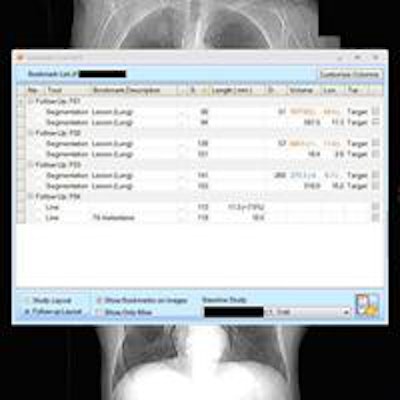
On the left, the lesion management application shows bookmarks, representing lesion descriptions, image/series numbers, and measurements that are exported from measurements made by the radiologist or radiologist extender. Once verified, these are translated to a RECIST report (right table and graphs) that instantly shows the tumor trajectory (in this case lesions getting larger) and supplements the traditional radiologist qualitative report. Image courtesy of Dr. Les Folio.
Ongoing development
In developments since the article was published, the NIH team is preparing for a software upgrade that will allow a quantitative oncologic reporting capability that their oncologist colleagues have been asking for, Folio said.
"Although we have a dedicated radiologist overseeing all tumor measurements and supplementing the traditional radiologist report, we plan on having several more radiologists making the tumor measurements at the time of dictation," he said.
This new capability will also require closer interaction with the referring teams, Folio noted. In an upcoming study, the researchers will survey their oncologists to help design their quantitative reporting system.
Another important advanced capability on the horizon is the ability to export annotation data into their hospital information system/electronic health record and clinical data management system/data warehouse, Folio said.
"We anticipate this will reduce transcription errors since pen-and-paper measurements and image and slice numbers are currently being typed into medical records and databases," Folio said. "This should provide timely quantitative reporting while saving time redigitizing already digitized information."
The group is also collaborating with other cancer centers to provide optimal data exchange of measurement data by complying with evolving standards such as the Annotation and Image Markup (AIM) standard and DICOM.
"This should allow images and corresponding centers to move to and from referring medical centers," he said.
New capabilities
A number of technical advances have also been developed since the study was completed, including lymph node segmentation, an "intelligent" 2D follow-up tool, volume-of-interest (VOI) analysis, and native reporting within PACS.
The lymph node segmentation capability is expected to be released as part of Caresteam's upcoming version 12 of the Vue PACS software, Folio said.
Designed to run in the background to assist radiologists who do not work with volumes, the intelligent 2D follow-up tool will only draw RECIST measurement lines.
"Whenever a study is marked graphically with a line or other graphic element, the tool will determine if another registered study has a graphic element present in a similar location, and if it finds one, [it will] automatically pair these two elements and report their growth rate," he said. "This may save more time, while alerting radiologists that a lesion has been measured before."
The NIH team is currently using another experimental version of the lung management application that utilizes VOI, allowing for assessment that is not limited to a traditional region of interest in an axial or other type of complex plane. The group is also using the VOI capability to assess what they are calling "total volume of viable tumor" for all lesions in a sarcoma and bladder cancer trial, Folio said.
"We hope this type of assessment will be comprehensive in that we are not just looking at a few select lesions," he said. "Although the task is monumental, we envision when automated segmentation of all normal and abnormal anatomy [will be] commonplace, with identifying/segmenting [of] all abnormal lesions."
In a prospective study, tumor growth models are also being applied as a supporting objective end point, and tumor necrosis is being compared with decreasing PET activity, Folio said.
The addition of native reporting will allow the radiologists to report these results directly within PACS, avoiding existing complicated integrations, advanced RIS capabilities, and third-party reporting software.
Folio also noted that in the experimental version of the software reported in the AJR study, study bookmarks were exported in tables and graphs to a RECIST report. In the next version, any bookmark in PACS will be accessible from Carestream's Vue Motion zero-footprint image viewer, allowing referring clinicians to rapidly navigate all findings with a single mouse click.
"We should strive to leverage developing advanced technologies to improve communication between radiologist and oncologists while helping to bring some 'life' and objectivity into traditional reporting," Folio concluded.