3D printing technology developer Stratasys said patient enrollment has begun in a clinical study that will assess the benefits of 3D-printed heart models in preoperative planning for pediatric heart surgery.
Known as 3D Hearts Enabling a Randomized Trial (3DHEART), the randomized, single-blind clinical trial will enroll 400 pediatric congenital heart patients requiring complex two-ventricle repair. Cardiopulmonary bypass time will be the primary end point in the study, while physician assessment of utility and the prevalence of morbidity and mortality will be secondary end points, Stratasys said.
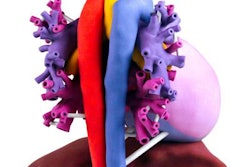
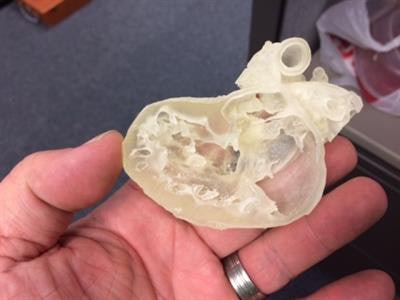
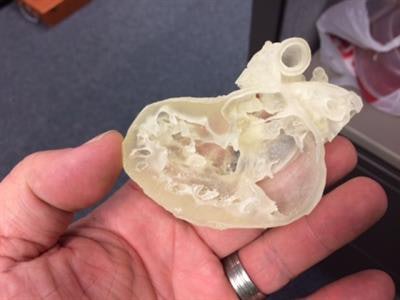 3D-printed pediatric heart. Image courtesy of Business Wire.
3D-printed pediatric heart. Image courtesy of Business Wire.Stratasys is providing in-kind support to the trial by providing heart models. Using its Connex 3D printers, the company's on-demand manufacturing unit will 3D print heart models for 200 patients based on their MRI or CT scans, enabling surgeons to evaluate and practice on a replica of the patient's heart prior to actual surgery, according to the firm.
Researchers will then compare the results of these 200 patients with 200 other patients who were treated without 3D models. The study is currently being led by physicians from NewYork-Presbyterian Morgan Stanley Children's Hospital in New York City; Children's Hospital of Philadelphia; Children's National Medical Center in Washington, DC; and Phoenix Children's Hospital. Up to 20 additional sites may also participate.
3DHEART is being managed by nonprofit organization OpHeart.