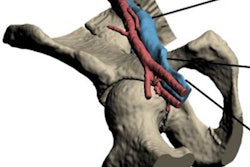
3D printing firm Materialise has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Mimics inPrint 3D printing software.
Materialise can now market the software to clinicians for use in U.S. hospitals for creating patient-specific 3D-printed models, according to the company. These models may facilitate preoperative planning and various diagnostic tasks, such as patient management, treatment, and surgeon-to-surgeon communication, Materialise said.