The U.S. Food and Drug Administration (FDA) has cleared patient-specific 3D-printed airway stents designed from CT scans by a physician from the Cleveland Clinic.
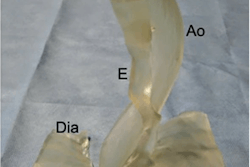
 Patient-specific 3D-printed airway stent. Image courtesy of Cleveland Clinic.
Patient-specific 3D-printed airway stent. Image courtesy of Cleveland Clinic.Dr. Tom Gildea and his team from the medical center used proprietary 3D visualization software and patient CT data to design virtual airway stents individually tailored to fit a patient's unique anatomy. They then used a 3D printer to generate the stents, into which they injected medical-grade silicone.
The patient-specific 3D-printed airway stent design offers a more precise fit that improves the longevity and comfort of stents compared with traditional silicone stents, the group said. Early in-house studies have shown that the patient-specific stents last about a year, on average, whereas traditional stock stents need to be replaced after about 90 days.
The group plans to establish a new company called VisionAir Solutions to provide the personalized stent design to medical institutions throughout the U.S. later this year.