Several image postprocessing methods are up for the task of assessing myocardial scar tissue from late gadolinium enhancement on cardiac MR exams, according to a multicenter study presented at the recent Society for Cardiovascular Magnetic Resonance (SCMR) meeting.
While the seven automated algorithms varied in their quantification of mean amount of scarred myocardium, a team from Johns Hopkins University judged a method based on image processing with background threshold correction (BCT) to be reliable for both ischemic and nonischemic scars. Two other automated methods were found to be suitable for nonischemic cases.
"In the setting of a multicenter clinical trial, [eight standard deviations above the mean remote myocardial signal intensity], BCT, and [full width at half maximum] automatic methods are the preferred methods for automatic quantification of ischemic scar, while only BCT is recommended for nonischemic scar," said Dr. Gustavo Volpe, who presented the findings at the San Francisco meeting.
Late gadolinium enhancement on cardiac MRI is the current noninvasive reference standard for assessing myocardial scar, and it has prognostic value for both ischemic and nonischemic cardiomyopathies. However, the standard methods used to determine scar quantification compromise reproducibility, according to Volpe.
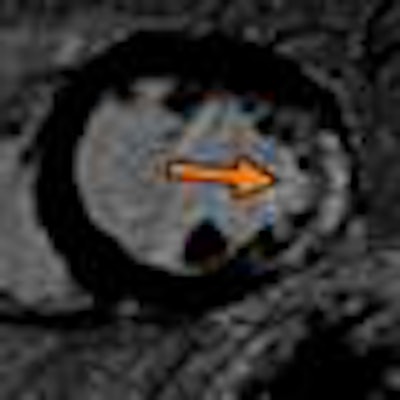
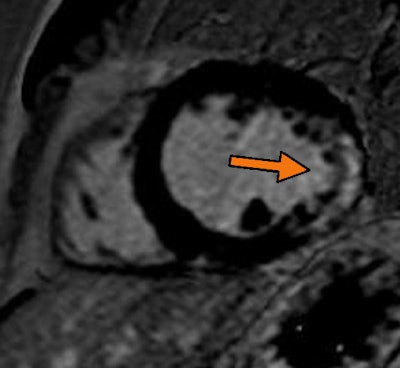 |
| Midchamber short-axis view from late gadolinium enhancement on cardiac MRI. Arrow shows transmural scar with an ischemic pattern at the later wall. Image from the Clinical Hospital of Ribeirão Preto in Brazil, courtesy of Dr. Gustavo Volpe. |
As a result, the researchers sought to evaluate the reliability of seven scar quantification techniques in the large, multicenter Multi-Ethnic Study of Atherosclerosis (MESA).
From the 2,805 total patients in MESA, the researchers included 1,661 patients who received late gadolinium enhancement cardiac MR studies on a 1.5-tesla scanner from either Siemens Healthcare or GE Healthcare at one of six centers. The patients ranged in age from 55 to 94.
Of the 1,661 patients, myocardial scar was visually detected in 137; 65 had ischemic cardiomyopathy, while 72 had nonischemic cardiomyopathy.
Of the patients with myocardial scar, 86% were male and mean age was 73 ± 9 years. Mean left-ventricular ejection fraction (LVEF) was 56% ± 6%, and mean left-ventricular mass was 158 g ± 37 g. Forty percent had more than 5% of left-ventricular vessel scar, according to Volpe.
The reference standard used in the study was the semiautomated method of visual computer-assisted planimetry with manual correction. In this method, imaging postprocessing is used to delineate late gadolinium enhancement planimetries using different automatic thresholds. The reader then chooses the best computed planimetry by visual assessment and manually corrects for partial volume and artifacts, Volpe said.
The researchers compared this method with six different automatic methods, including two, four, six, or eight standard deviations above the mean remote myocardial signal intensity (SI); full width at half maximum (FWHM); and background correction threshold (BCT).
BCT calculates the threshold for each individual slice based on the sum of the mean signal intensity of the entire myocardium, two standard deviations of the remote myocardium, and two standard deviations of a region-of-interest placed in the "air" in each slice, according to the researchers.
The team assessed inter- and intraobserver agreement and reproducibility by paired t-test, concordance correlation coefficient, and Bland-Altman analysis. The researchers used the research version of the QMass MRI quantification software from Medis Medical Imaging Systems in the study.
The mean amount of scarred myocardium varied between methods, Volpe and colleagues found. However, no significant differences were found between the reference standard and eight standard deviations above the mean remote myocardial SI (p = 0.98), FWHM (p = 0.17), or BCT (p = 0.51) techniques in the ischemic group. In nonischemic cases, eight standard deviations above the mean remote myocardial SI (p = 0.32) and BCT (p = 0.42) did not differ significantly with the semiautomated reference standard.
The two, four, and six standard deviations above the mean remote myocardial SI overestimated the amount of scar by factors of 2.6, 1.8, and 1.3, respectively, in ischemic cases, and by factors of 5.2, 3.1, and 1.9 in nonischemic cases, according to Volpe. In addition, FWHM overestimated scar by a factor of 2.2 and eight standard deviations above the remote myocardial SI overestimated scar by a factor of 1.8 in nonischemic cases.
All methods yielded excellent reproducibility, with a concordance correlation coefficient (CCC) ranging from 97% to 99%.
The authors acknowledged the limitations of their research, including the lack of histopathology evaluation and their approach of a population-based study with small scar areas. In addition, the multicenter study used different vendors, with five Siemens scanners and one GE system, Volpe said.