A new postprocessing technique for dual-source CT colonography (CTC) corrects for artifacts that mimic contrast enhancement created by fecal tagging materials. The algorithm boosts sensitivity and reduces false-positive detections significantly.
Called IPC2, the technique was developed at Massachusetts General Hospital (MGH) for use with single-source CTC; however, MGH researchers are finding that it is particularly useful with dual-source CT because the modality enables the use of virtual monochromatic images to separate materials in the colon. The IPC2 algorithm eliminates pseudoenhancement artifacts, which are caused by artificially high attenuation in areas adjacent to materials tagged with contrast agents.
And, importantly, IPC2 corrects for distortions that pseudoenhancement can cause to radiomic information, or data related to quantitative imaging features such as effective atomic number, dual-energy index, and material density maps. Radiomics is an increasingly important component of image analysis in advanced modalities.
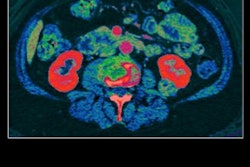
Artifacts from dual-energy CT
 Janne Nappi, PhD, from MGH and Harvard Medical School.
Janne Nappi, PhD, from MGH and Harvard Medical School.
Once the algorithm is applied, "all the quantitative radiomic information obtained from the dual-energy images will be corrected as well," explained Janne Nappi, PhD, from MGH and Harvard Medical School.
Nappi and colleagues Hiroyuki Yoshida, PhD, and Dr. Se Hyung Kim set out to evaluate the effect of IPC2 on polyp detection and false positives in dual-source CTC. Previously, a similar technique the group designed for single-source CTC had improved results significantly, Nappi explained in a talk at the December RSNA 2015 meeting in Chicago.
"Untagged fluid and feces in the colon can imitate colorectal lesions, and therefore a contrast agent is administered orally to opacify the fluid and feces in the CTC images," he said. "The problem is that these high-density concentrations of fecal tagging artificially elevate observed CT values of adjacent materials -- then lesions can become invisible with the usual CT display window settings."
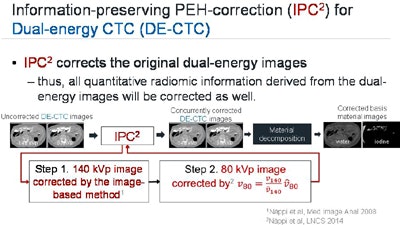 Flow chart shows that the IPC2 algorithm corrects the original dual-source CT images after high-density concentrations of tagging material artificially affect attenuation. Radiomic information is corrected along with the image data and subsequent material decomposition images. Image courtesy of Janne Nappi, PhD.
Flow chart shows that the IPC2 algorithm corrects the original dual-source CT images after high-density concentrations of tagging material artificially affect attenuation. Radiomic information is corrected along with the image data and subsequent material decomposition images. Image courtesy of Janne Nappi, PhD.To test the algorithm, the researchers examined 20 patients who underwent low-dose dual-source CT after one-day bowel preparation, measuring its performance with a previously validated computer-aided detection (CAD) scheme. Sensitivity was markedly improved -- from about 71% to 82% for large polyps -- when IPC2 was activated.
"For all clinically meaningful lesions -- all polyps, large polyps, and small polyps -- the improvement in detection accuracy ... was statistically significant," Nappi said.
The MGH investigators applied IPC2 retrospectively to the cohort, whose one-day bowel preparation consisted of 18 g of magnesium citrate.
All images were acquired on a dual-source CT scanner (Somatom Flash, Siemens Healthcare) at 140 and 80 kVp, with collimation of 64 x 0.6 mm, slice thickness of 1 mm, and an estimated CT dose index volume (CTDIvol) of 2.5 mGy per position, prone or supine. Filtered back projection was used for reconstruction.
The investigators performed fecal tagging using 60 mL of nonionic iodine; no intravenous contrast was used. An experienced, board-certified radiologist correlated the CTC images with the findings of subsequent optical colonoscopy, Nappi said.
The data from 20 patients included 29 biopsy-confirmed colorectal lesions, with 14 large polyps (≥ 10 mm) and 15 small polyps (6-9 mm). Three were carcinomas and 10 were adenomas. A majority of lesions had tagging material on the surface, but 45% had no tagging and 21% were mostly covered by fecal tagging.
For assessment, the group used a leave-one-patient-out (LOPO) method to evaluate the detection performance of dual-energy CAD both with and without the IPC2 algorithm. They assessed the results using free-response receiver operator characteristics (FROC) curves.
Better detection, fewer false positives
The performance of the CAD algorithm was higher with the use of IPC2 than without it, Nappi said. Using IPC2, sensitivity was 86%, with an average of six false-positive detections per patient. Without IPC2, sensitivity was 73%, with about seven false positives per patient.
The researchers also assessed the performance of the IPC2 algorithm by lesion size, as measured by area under the free curve (AUFC) at the 95% confidence interval.
| Performance of IPC2 algorithm by lesion size | |||||
| Lesion size | With IPC2 | Without IPC2 | |||
| ≥ 6 mm | 0.84 | 0.71 | |||
| ≥ 10 mm | 0.83 | 0.74 | |||
| 6-9 mm | 0.63 | 0.52 | |||
The results show improved lesion detection with use of the IPC2 algorithm to eliminate pseudoenhancement.
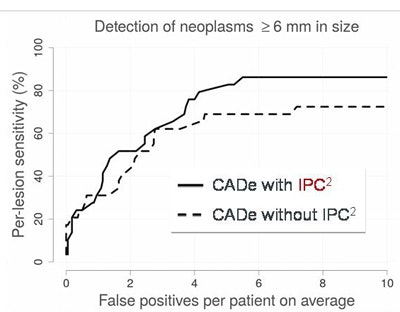 LOPO-FROC curve shows that IPC2-corrected images reveal higher sensitivity with a lower false-positive rate for colorectal lesions in CTC, compared with uncorrected image data with tagging artifacts. Image courtesy of Janne Nappi, PhD.
LOPO-FROC curve shows that IPC2-corrected images reveal higher sensitivity with a lower false-positive rate for colorectal lesions in CTC, compared with uncorrected image data with tagging artifacts. Image courtesy of Janne Nappi, PhD."Pseudoenhancement can corrupt radiomic information in dual-source CTC, and this can also happen in regular CTC," Nappi said. "The IPC2 method can be used to correct for pseudoenhancement and correct all the radiomic images' data."