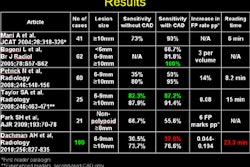
Computer-aided detection software developer Riverain Technologies has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Temporal Comparison software.
By electronically comparing current and prior chest x-ray images, the software can help identify nodules that may be early-stage lung cancer, according to the vendor. It aligns and registers two patient images to produce a difference image, which enables radiologists to pinpoint subtle changes, Riverain said.
Temporal Comparison has also received marketing clearance in the European Union.