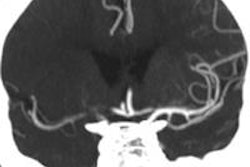
The U.S. Food and Drug Administration (FDA) said it has allowed the marketing of two catheter-based clot retrieval devices from medical device company Concentric Medical as an initial therapy to reduce paralysis, speech difficulties, and other symptoms of ischemic stroke.
The Trevo devices should be used within six hours of symptom onset, and only after treatment with tissue plasminogen activator (tPA), which must be given within three hours of symptom onset, the FDA said.
The FDA first cleared the Trevo device in 2012 to remove blood clots in patients with contraindications to tPA or who did not respond to the therapy. The current action expands the indication of the devices, according to the agency.