CHICAGO - A new dual-energy CT mode, an MRI elastography technique, and a cost-effective mammography system are among the highlights in the RSNA booth of GE Healthcare of Chalfont St. Giles, U.K.
CT
In CT, GE is spotlighting new capabilities for its Discovery CT750 HD scanner, including a dual-energy imaging mode and radiation dose-reduction techniques.
GE launched CT750 HD in 2008, touting the scanner's Gemstone detector design, which uses a garnet-based detector material. At this year's show, the company is rolling out Gemstone spectral imaging, a dual-energy imaging technique that's based on the Gemstone material.
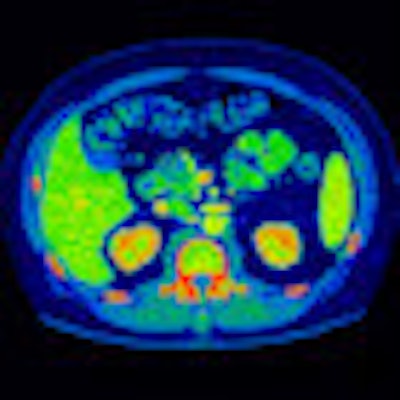
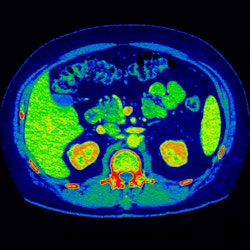 |
| Image acquired with GE's Gemstone spectral imaging technique displays MD iodine color map that suggests renal lesion enhancement. All images courtesy of GE. |
The technique uses what GE calls fast kVp switching, in which the kVp levels are switched with every other view -- a capability made possible by Gemstone detector material. A special viewing mode enables users to scroll through a stack of images to view different keV levels.
Gemstone spectral imaging began shipping in October, and the company has installed the technology at 20 sites.
GE is also talking up its adaptive statistical iterative reconstruction (ASIR) technique, which reduces radiation dose between 30% and 50% by replacing the filtered back projection technique traditionally used in CT with an iterative reconstruction mode. ASIR was initially available on the CT750 HD scanner, and GE is now rolling it out on its 64-slice LightSpeed VCT system as well. The company also plans to make ASIR available internationally on the BrightSpeed 16-slice scanner.
GE is already working on the next generation of ASIR, called model-based iterative reconstruction (MBIR). Like ASIR, MBIR will require additional computing horsepower for the scanners that use it, but GE expects MBIR to achieve additional dose reduction comparable to that achieved by ASIR compared to filtered back projection reconstruction.
GE has launched a multicenter clinical study to investigate MBIR, with the goal of determining the level of performance that can be achieved with ultralow-dose CT for a variety of conditions in the brain, chest, and abdomen.
MRI
Taking center stage in the MRI section of GE's booth is Optima MR450w, a new scanner launched earlier this year sporting a 70-cm bore diameter. The company is highlighting a new elastography application for the scanner, called MR-Touch.
 |
| Optima 450w is an MRI scanner with a 70-cm internal bore diameter. |
Elastography is better known as an ultrasound technique, but GE has developed a version for MRI by attaching a small box-sized generator next to the scanner that transmits ultrasound pulses into a patient's abdomen during MR scanning. The sound waves reflect off tissue differently based on stiffness, with malignant tissue having a different stiffness profile than healthy tissue. Color-coded images are then produced that detail the different stiffness levels.
GE plans to roll out MR-Touch initially on Optima MR450w, then on scanners in the company's HDx line. The application has 510(k) clearance.
Another highlight of GE's booth is Optima MR360, a 1.5-tesla superconducting MRI scanner that the company plans to sell for less than $1 million. GE has been able to cut production costs for the system by manufacturing it at a GE plant in China, although the scanner uses a magnet made in the U.S.
GE believes the system will be a primary MRI scanner for many international customers, and in the U.S. it could be a second or third scanner at many sites, or even a primary scanner for sites wanting new MRI technology in an era of healthcare reform.
MR360 has a 60-cm bore, with gradients rated at an amplitude of 33 millitesla/meter and slew rate of 100 tesla/meter/second. The scanner is pending 501(k) clearance, and shipments are planned for the second quarter in the U.S. It is already shipping in some countries where it has received regulatory approval.
Other new developments in GE's booth include new clinical applications, such as Propeller 3.0 for motion compensation and 3D ASL (arterial spin labeling), an automated brain perfusion technique. GE's HDxt scanners are also receiving a new suite of applications.
Finally, GE is highlighting the dedicated extremity MRI scanners it acquired through its purchase of assets from ONI Medical Systems. GE has branded the scanners as ONI MSK Extreme 1.5T and ONI MSK Extreme 1.0T.
Women's imaging
Senographe Essential e is a new full-field digital mammography (FFDM) system that GE is launching at this week's meeting as a cost-effective platform exclusively for screening applications.
 |
| Senographe Essential e is a new FFDM system optimized for breast screening. |
GE eliminated a number of options on its Senographe Essential platform to keep production costs down with the new system, such as dispensing with compression paddles used in diagnostic mammography. The company believes that Essential e is a good option for sites that already have a full-featured Essential system for diagnostic work.
Essential e units began shipping this quarter, according to the company, and purchasers can also upgrade to the full-featured Essential should their needs change in the future.
Meanwhile, TechInsight is a portable console that is designed to help improve workflow in a mammography suite by enabling technologists to perform tasks such as image annotation while keeping the main console free. It can be based on a tablet PC, laptop, or mobile cart and can be networked to a PACS to pull up prior images. TechInsight does not have 510(k) clearance and is being shown as a work-in-progress, although it is shipping in Europe.
For GE's Senographe Essential Interventional FFDM system, the company is showing a biopsy table in which patients are positioned decubitus rather than prone. GE believes that this provides users with more positioning flexibility than a standard prone table.
Finally, GE announced that it is planning to use Senographe Essential as the platform for the digital breast tomosynthesis (DBT) system it is developing. When DBT becomes commercially available, Senographe Essential users in the field will be able to upgrade to the new technology. GE said it is currently working with the U.S. Food and Drug Administration (FDA) on a regulatory application for DBT.
Molecular Imaging
GE is highlighting an enhanced application suite for its Discovery PET/CT 600 series in its booth. All systems in the platform can now utilize GE's Vue Point HD reconstruction technique, MotionFree imaging technology, and reconstruction engine, according to the vendor.
The company is also displaying its Discovery PET/CT 690 system with BrightSpeed Elite CT, a combination GE believes confers advanced imaging and application capabilities.
In addition, GE is emphasizing the dual-detector capabilities of the 600 series. For example, a Discovery PET/CT 600 scanner can be configured with a Discovery PET/CT 690 detector, allowing for flexibility in clinical implementation and imaging exploration, GE said.
In service enhancements for the 600 series, GE has debuted InSite OnWatch for PET/CT, allowing for data-driven prediction tools to monitor systems, identify impending failures, and forecast maintenance needs, the company said.
Thanks to the use of an improved IBM Blade Center, GE said its Vue Point FX reconstruction tool is now twice as fast, requiring less than 75 seconds per bed position. In addition, a 2-meter scan option allows clinicians to perform an entire head-to-toe examination in one scan, GE said.
GE is also directing attention to the use of an ASIR technique on its Discovery PET/CT 690 system with LightSpeed VCT. The technique has reduced CT dose by up to 40% and improved low-contrast detectability (LCD) by 30%, the company said.
SharpIR, an iterative reconstruction enhancement to Vue Point HD and Vue Point FX, provides enhanced visual contrast and resolution on both whole-body and brain PET images, GE said.
GE also said it would install its first Discovery NM/CT 670 scanner for clinical use at Rambam Hospital in Haifa, Israel. The company introduced the hybrid imaging scanner in October at the 2009 European Association of Nuclear Medicine (EANM) meeting in Barcelona, Spain.
Discovery NM/CT 670 is currently pending FDA 510(k) clearance.
Ultrasound
In the ultrasound section of the company's booth, GE is highlighting its Breakthrough 2010 upgrade on its Logiq 9 general imaging ultrasound scanner.
The release features seven new E-Series transducers and a number of software enhancements aimed at providing image quality and productivity gains for pediatric, vascular, and cardiac imaging, according to GE. A new cardiac tool suite has also been included.
Vascular enhancements include Auto IMT (intima media thickness) capability, while the cardiac tool suite adds feature such as Q-Analysis, Tissue Velocity Imaging, CW (Continuous Wave), ECG, and color M-Mode, according to GE.
GE also unveiled the first system in its new Venue miniaturized ultrasound line. Venue 40 features a touchscreen interface and is targeted for bedside use by clinicians or for guiding interventional radiology procedures.
The company is also showcasing new features on its Logiq P6 general imaging system, including advanced image optimization algorithms for improved 2D image quality, real-time 4D upgrades, new transducers, a new hard drive, expanded reporting packages, and an enhanced 17-inch monitor, GE said.
In other ultrasound highlights, GE is presenting its Breakthrough 2009 upgrade for Voluson E8. The scanner has received a new dynamic rendering engine for improved 3D rendering, as well as enhancements such as the firm's OmniView fetal cardiac volume contrast imaging capability and Scan Assistant tool for quality assurance and workflow improvement.
GE also unveiled a Breakthrough 2010 release for its ViewPoint ultrasound reporting and image management offering. The new release brings a new thyroid reporting capability and a vascular reporting update, according to the vendor.
The company has also incorporated support for cluster servers, which allow users to run two separate servers for increased availability, GE said. In addition, a third-party HIS, RIS, and PACS interface has been added.
X-ray
In x-ray, GE is highlighting a brace of systems introduced at last year's meeting, Discovery XR650 for advanced applications and Optima XR640 for routine use. Both units began shipping in the second half of this year.
GE is highlighting the growing number of clinical studies based on its VolumeRad digital tomosynthesis option for Discovery XR650, with some researchers investigating whether it can be used as a less costly (and less dose-intensive) alternative to CT. Also prominent is dual-energy subtraction, which can separate bone from soft tissue to give users a better look at each.
In mobile imaging, the company is also touting a milestone for its OEC 9900 Elite mobile C-arm, with more than 2,300 units shipping in the past 18 months. GE said the figure represents customers who waited to order GE C-arms while the business unit was unable to ship products due to a consent decree the company entered with the FDA. GE would normally ship 1,500 to 1,700 units in an 18-month period.
In interventional radiology, GE is launching Innova 4100IQ, an angiography system that represents the next generation of the company's angiography technology. The system includes applications such as subtracted 3D to better visualize vessels without having to remove surrounding bone, and blended roadmap, which superimposes a digital subtraction angiography (DSA) or InnovaBreeze bolus image with fluoroscopy to streamline workflow.
Innova 4100IQ uses a 40 x 40-cm flat-panel digital detector and a new Advantage Workstation VolumeShare 4 computer. Tableside controls also give users more flexibility in operating the system.
Finally, AutoEx is a new dose-control system for the Innova product line that automatically and continuously adapts to keep patient dose as low as possible. GE believes AutoEx can reduce dose by as much as 40% without compromising image quality.
Healthcare IT
GE is directing attention to version 3.7.3 of its Web-based Centricity PACS-IW software. With the new release, customers all over the world can use Centricity PACS-IW, featuring systems integration capability as well as language and character set localization in English and ten additional languages, GE said.
Other 3.7.3 features include various performance enhancements, an improved user interface, and optimized integration with GE's Centricity RIS-IC and Centricity Precision Reporting, according to the firm.
The new release also includes the vendor's optional Oncology Workflow module, first shown at the 2008 RSNA meeting. Oncology Workflow provides PET/CT image fusion, reconstruction, and automatic maximum intensity projection (MIP) within the PACS user interface, GE said.
GE also said that its Web-based Centricity PACS-IW offering has now been deployed at 100 international institutions and more than 400 sites in the U.S. Recent international customers include Kurume University School of Medicine in Kurume, Japan; Diwan, Chand & Arrgarwal (DCA) in New Delhi, India; and Hospital Universiti Sains Malaysia (HUSM) in Kuala Lumpur, Malaysia.
Version 3.7.3 is also available in a Web-based RIS/PACS configuration as Centricity Radiology-IW, including integration with RIS-IC and Precision Reporting.
In other developments, GE is touting its Centricity OneView Global Reading application, which allows users to access images and information from disparate and multivendor RIS/PACS on a single worklist.
A sister OneView product, OneView Single Patient Jacket, provides access to patient records across disparate systems. Single Patient Jacket has been installed in the Southwestern Ontario Diagnostic Imaging Network in Canada, integrating 19 PACS and 12 RIS multivendor systems across more than 30 hospitals, GE said.
In other healthcare IT developments, GE has integrated its Precision Reporting speech recognition tool with Centricity PACS. This allows for GE to apply its speech "understanding" engine to capture patient data throughout the radiology reporting and image archiving processes, according to the firm. The company initially showed Precision Reporting as an embedded application within RIS-IC at last year's meeting.
By Brian Casey and Erik L. Ridley
AuntMinnie.com staff writers
November 30, 2009
Related Reading
GE to debut MR elastography, November 24, 2009
GE completes ONI acquisition, November 20, 2009
GE touts ADMIRE-HF clinical results, November 17, 2009
GE inks European Vscan evaluation deal, November 5, 2009
GE awarded $1.2M NIH grant, November 3, 2009
Copyright © 2009 AuntMinnie.com