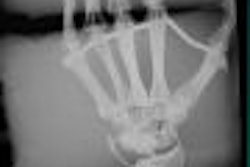
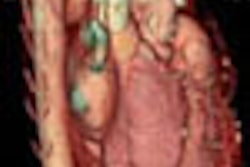
Siemens Medical Solutions has received Food and Drug Administration 510(k) clearance for Arcadis Avantic, a mobile C-arm system.
The system is designed for advanced imaging requirements in trauma and spine surgery, general surgery and urology, orthopedic surgery and pain management, gastroenterology, and vascular surgery, according to the Malvern, PA-based vendor. It includes features such as 20 kW of power, up to 250 mA output, and a 13-inch image intensifier.
The unit employs a digital imaging chain that generates and manages all image data at a resolution of 1024 x 1024 pixels, Siemens said. Two 18-inch flat-panel monitors display images and results in digital cine mode up to 250 mA, and film sequences are acquired and stored at speeds up to 30 frames per second.
Siemens expects to begin shipping the unit in September.
By AuntMinnie.com staff writers
August 15, 2005
Related Reading
Siemens hooks up with Xilinx, August 2, 2005
Siemens Medical grows sales and profit, July 28, 2005
Siemens makes $1.5 million donation, July 26, 2005
Siemens hits Axiom Artis dBC milestone, June 27, 2005
Siemens hits 120 milestone with Espree, June 22, 2005
Copyright © 2005 AuntMinnie.com