CHICAGO -- SPECT/CT imaging during lutetium-177 prostate-specific membrane antigen-617 (Lu-177 PSMA-617) treatment may help identify prostate cancer patients at higher risk of poor outcomes, according to research presented December 1 at the RSNA meeting.
Presenter Yalda Nikanpour, MD, a postdoctoral research fellow at the Mayo Clinic in Rochester, MN, and colleagues, found that quantitative changes in tumor burden on SPECT/CT were linked to overall survival in patients.
 Yalda Nikanpour, MD, presented findings on SPECT/CT imaging in cancer patients receiving Pluvicto on December 1 at RSNA in Chicago.
Yalda Nikanpour, MD, presented findings on SPECT/CT imaging in cancer patients receiving Pluvicto on December 1 at RSNA in Chicago.
Lu-177 PSMA-617 (Pluvicto, Novartis) was approved by the U.S. Food and Drug Administration (FDA) in March 2022 for adults with prostate-specific membrane antigen (PSMA)-positive metastatic cancer who have not responded to standard treatments. While the drug benefits patients, individual outcomes vary, and reliable survival predictors are lacking, Nikanpour noted.
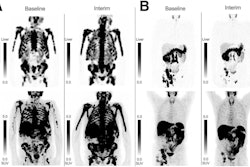
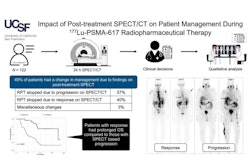
Hence, the researchers analyzed post-therapy whole-body SPECT/CT imaging performed 24 hours after each infusion among 50 patients with metastatic castration-resistant prostate cancer. Five patients received four cycles, 12 received five cycles, and 33 completed six cycles of therapy, with a mean overall survival (OS) for the cohort of 24.7 months. The researchers calculated cycle-to-cycle changes in PECT/CT quantitative metrics.
According to the analysis, quantitative changes in tumor burden were associated with overall survival, particularly at cycles four and five, Nikanpour said. The strongest association was observed at cycle four, where change in lesion (radiotracer) uptake volume from baseline was linked to predicted survival (hazard ratio [HR], 3.1; p = 0.0009). In addition, total lesion volume changes from baseline to cycles four and five also reached significance for predicting survival (HR, 2; p = 0.009).
Finally, lesion maximum standardized uptake value was predictive only at cycle five (HR 1.7, p = 0.019), but predicted uptake independently of tumor volume at this cycle, according to the findings.
“Quantitative 24-hour post-therapy Lu-177 PSMA-SPECT/CT provides early prognostic information during treatment,” Nikanpour said.
Ultimately, the variation in how patients respond to treatment with Pluvicto -- some achieve durable control and others progress early -- has created a need for early markers to predict survival. Standard clinical measurements of prostate-specific antigen (PSA) may not reflect full disease activity, given that some patients progress despite favorable PSA changes, she added.
“Early imaging markers may provide stronger and more reliable indicators of survival,” Nikanpour concluded.
For full coverage of RSNA 2025, visit our RADCast.