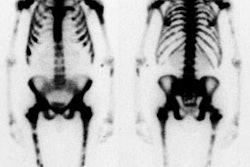
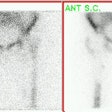
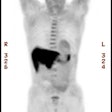
Ventilation/Perfusion Imaging:
Technique & Radiopharmaceuticals:
Perfusion Agent:
The agent used for perfusion imaging is Tc-99m macroaggregated albumin (Tc-99m MAA). Particle size is generally between 10 to 90 microns (90% of particles) and no particles should be larger than 150 microns. Tc-99m MAA is injected slowly IV and lodges in percapillary arterioles, obstructing approximately 0.1% of their total number. The particles clear by enzymatic hydrolysis and are phagocytized by RE cells (the agent has a biologic half-life in the lungs of between 6-8 hours). Normally, only 3 to 6% of the injected Tc-99m MAA will bypass the pulmonary vasculature. The critical organ is the lungs which receive a dose of about 1 rad (1 cGy) from a typical 5 mCi dose. The kidneys and bladder receive moderate exposure largely from the excretion of degraded albumin.
A 5 mCi dose usually contains on average 500,000 [8]. The arterioles are only temporarily occluded because the material degrades into smaller particles which are eventually phagocytized by cells of the reticuloendothelial system [8]. The biological half-life of MAA in the lungs is about 6 to 8 hours [8]. The number of particles used for the exam should be reduced to about 100,000 in patients with pulmonary arterial hypertension, and in those with known right to left shunts. To label a smaller number of particles while maintaining the dose of Tc-99m, the vial should be reconstituted with a larger amount of activity. A given number of mCi then represents a smaller portion of the total vial, and thus contains fewer particles. A minimum of 70,000 particles is necessary to obtain a diagnostic quality scan in an adult. It is estimated that neonates have about 10% of the eventual number of adult pulmonary capillaries. In neonates, the number of particles should be reduced to between 10,000 to 50,000 to maintain an adequate margin of safety. The number of capillaries increases to half of the adult value by age 3 years, and reaches an adult level by age 8 to 12 years. A dose should be administered only if the preparation is less than 6 hours old. This is because Tc-99m MAA tends to aggregate and Tc-99m slowly dissociates from Tc-99m MAA over time. Also, decay of the radiopharmaceutical over time results in the need for a larger volume (and hence greater number of particles) to obtain the same dose. For imaging, a large field of view camera with a parallel hole, high-resolution collimator should be used. Images should contain at least 750,000 counts.
Ventilation Agents:
Xenon-133 (Beta-minus decay):
Xe-133 is a fission product of U-235. It has a physical half-life of 5.24 days and a low energy gamma emission (81keV) that will be rapidly attenuated by overlying tissue; therefore, scanning is performed posteriorly. The typical dose used for the exam is 15-25 mCi, most commonly inhaled, but can be administered in saline IV. The critical organ is the trachea and airways which receive about 100 mrad/mCi/liter/min (total dose to the trachea is approximately 28 mrad from inhalation and 11 mrad following IV injection [R]). Less than 15% of the dose is absorbed by the body. The Xe-133 ventilation scan is generally performed prior to the Tc-99m MAA perfusion exam, as downscatter from the Tc-99m would severely degrade image quality. Some centers perform the Xenon exam after the perfusion study by administering a very low dose (1-2 mCi) of Tc-99m MAA and a much higher dose of Xenon-133 (20-30 mCi). A narrow window setting (10%) on the Xenon-133 gamma energy is also applied to reduce the contribution from scatter. Images can then be obtained in the projection which best demonstrates the perfusion defects.
Computer subtraction techniques have been developed to correct for Tc-99m scatter in the Xenon-133 energy window, but the method is difficult to apply and not routinely used.
The gamma camera is placed behind the patient's back and a bolus of 15-20 mCi of Xe133 is injected into the mouthpiece of the spirometer system at a time when the patient begins maximal inspiration [8]. The xenon exam consists of 3 phases:
- Single breath: The patient takes a single deep inspiration and holds it for as long as possible. This view reflects regional ventilation and can detect approximately 66% of ventilation defects associated with obstructive airway disease.
- Equilibrium: During this phase, the patient performs normal tidal respirations for at least 3 minutes, and 5 minutes if possible, while rebreathing a mixture of Xenon-133 and oxygen. This view demonstrates the overall lung volume as tracer equilibrates between all aerated portions of the lungs. It is the least sensitive for detecting obstructive airway abnormalities. However, adequate duration of rebreathing ensures diagnostic quality washout phase.
- Washout: Inhalation of Xenon-133 is discontinued and the patient breaths room air or oxygen while exhaling the xenon into a charcoal trap. Xenon-133 should clear from the lungs normally in 2-3 minutes. Retention of tracer activity beyond 3 minutes is observed in areas of air trapping (obstructive airway disease). The washout phase of the exam will detect about 90% of abnormalities associated with obstructive airway disease (it is the phase of the exam that is most likely to show ventilation abnormalities [8]). It is therefore more sensitive than the single breath view in this regard. Posterior oblique views may also be obtained to improve spatial localization of lung zones with slow washout [8]. Xenon is overall more sensitive than aerosolized Tc-DTPA for detecting obstructive lung disease.
Xenon 127 (Electron capture):
Xe-127 is cyclotron produced and has a longer physical half-life (36.4 days) than Xe-133. Its gamma emissions: 172 keV (25%), *203 keV (68%), and 375 keV (18%) are of higher energy than 140 keV emission of Tc99m. It can be used post-perfusion to evaluate specific defects. The higher photon energies require the use of a medium or high energy collimator. Additionally, the xenon trap system must be more heavily shielded and stored for a considerably longer period of time prior to adequate decay for disposal.
Tc-99m DTPA Aerosol:
Using an aerosol delivery system that generates submicronic particles, 30 mCi of Tc-DTPA in 3ml of saline (3 to 5 minutes of rebreathing on the system with the oxygen at 8 to 10 liters/min.) delivers about 500 to 750 uCi of tracer to the lungs [8]. This dose yields 100K counts images in about 2 minutes on a standard gamma camera with a low energy general purpose collimator [8]. The typical radiation exposure to the lungs is about 100 mrads. This is less than the several hundred millirad exposure from a typical Xe133 rebreathing ventilation exam. The dose to the lungs is also less than that from a Kr-81 exam [8]. Images should be acquired for 100k counts or 5 minutes. Exposure to personnel is usually less than that delivered during a Xenon study.
Nebulizers produce particles that are generally between 0.5 to 0.8 microns in diameter. Because of their small size, these particles can be delivered to the alveoli like gases during inhalation. Deposition of particles larger than 1 micron in the tracheobronchial tree is influenced primarily by inertial impaction and gravitational sedimentation. Even larger particles will deposit more proximal in the airways. Benefits of this procedure are that collection devices are not necessary as they are for radioactive gases, and persistence of activity in the lungs permits images in multiple projections to be obtained. Disadvantages are the inability to demonstrate areas of air trapping and excessive tracer is often seen in the proximal bronchial tree in patients with obstructive airway disease due to turbulent airflow. Tachypneic patients also tend to have prominent central deposition of the tracer.
Radioaerosol imaging studies can be performed in patients on ventilator assistance, but to avoid central tracer deposition from turbulent air flow, the ventilator should be set to the lowest peak flow rate possible and it is best to turn off PEEP during the inhalation portion of the study. Nonetheless, the correlation between regional ventilation and aerosol deposition is poor in ventilated patients and better definition of ventilated segments can be obtained by using a gas [9].
The ventilation portion of the exam may be performed before or after the perfusion study. If it is done after the Tc-99m MAA exam, the dose placed in the nebulizer should be increased to 45 mCi (or more) and the inhalation of the aerosol should continue until the count rate obtained is 3 times the count rate of a posterior Tc-99m MAA image in order to overwhelm the activity present in the lungs (perfusion defects will "fill-in" with activity in areas of pulmonary embolism). Most authorities, however, believe that Tc-DTPA aerosol images should be obtained before the Tc-99m MAA exam.
Over time, aerosolized Tc-DTPA crosses the alveolar-capillary membrane and enters the bloodstream where it is then filtered by the kidneys and excreted. The critical organ is the urinary bladder. In normal patients, the pulmonary clearance of Tc-DTPA has a half-time of over 60 minutes (1 to 1.5 hours). The disappearance rate from the lungs has been found to be more rapid in patients with pulmonary embolism (affected lung segments primarily), lung injury (ARDS), pulmonary fibrosis, and in smokers (as rapid as 20 minutes). Aerosolized Tc-PYP has a significantly slower clearance rate than Tc-DTPA in both smokers and non-smokers.
Tc-99m Technegas:
Tc04 is vaporized in a microfurnace to produce ultrafine labelled carbon particles. The material is produced by heating 5mCi of Tc-pertechnetate to very high temperatures (2500 degrees Celsius) in a crucible in the presence of 100% argon gas. A soot or ash material is produced which is thought to be a Tc-carbon particle that is so small it acts like a gas. The median size of the technegas particles is between 0.05 and 0.15 microns, and there is good peripheral deposition even in patients with COPD. Since the material is produced in argon, inhalation may cause transient hypoxemia; this can be overcome by giving oxygen via nasal canula. No severe adverse reactions have been reported to date. There is longer pulmonary retention of Technegas with no effective clearance (the clearance half-time is 6 hours- the physical half-life of Tc-99m). Since the material produced is not filtered and contains up to 50% of the initial radioactivity, a large number of appropriately sized particles are inhaled with each breath. Thus, only a few inspirations (typically 2 to 10) are needed to reach an adequate dose. Usually about 1 mCi is deposited in the lung. Extrapulmonary activity in the oropharynx, trachea, and stomach can be seen in about 30% of patients. The exam may be technically inadequate in up to 15% of patients [4]- particularly in severely ill patients that cannot be instructed for inhalation, or in patients with very shallow or rapid breathing [4]. If the Technegas portion of the exam is performed following the perfusion study, a counting rate of at least two times the count rate of the perfusion exam is considered adequate [4]. Remember, if the inhaled activity is insufficient, the perfusion distribution will continue to dominate the final images [4].
If the reaction is produced in an atmosphere which contains 2 to 5% oxygen, a different agent is produced called `Pertechnegas,' which is a vapor of pertechnetate. This agent also demonstrates excellent distribution in the lungs, but it is absorbed relatively rapidly (half time clearance from the lungs is 7 to 10 minutes [5]), and repeat administration of the agent may be necessary during the exam.
Krypton-81m (Isomeric transition):
Produced from a Rubidium Krypton-81m generator. Krypton-81m decays by isomeric transition, with a gamma emission of 191 keV (65%), and a physical half-life of only 13 seconds [8]. Because it is not a nuclear byproduct material and it decays so rapidly to relatively stable Krypton-81, Krypton-81m is not considered an environmental hazard and storage/collecting devices are not necessary. As the energy is higher than Tc-99m, and the physical half-life is so short, ventilation images are usually recorded immediately after each perfusion view without moving the patient. This provides an exact comparison of ventilation and perfusion. Regions of abnormal ventilation are scintigraphically portrayed as areas of diminished or absent activity. In effect, the radioactive gas decays before it reaches the small airspaces of poorly ventilated regions. Because physical decay occurs before an equilibrium distribution can be attained, washout information regarding obstructive lung disease cannot be obtained and some zones of mild obstructive disease may not be detected. The radiation dose to the lungs is significantly lower than with other agents (about 15 mrad per view). Because the parent isotope Rubidium 81m has a physical half-life of about 4.5 hours, the use of the generator is usually 1 working day which makes it impractical for most clinical situations.
Modifications to the V/Q exam:
Decreased Particle Count:
A decreased number of particles (approximately 100,000) should be used when performing perfusion imaging in some patients:
1- Severe pulmonary hypertension:
These patients have a limited pulmonary vascular reserve, and acute right heart failure may occur. Death has been reported in these patients immediately following injection of a standard dose of particles.
2- Right to Left shunt:
Will see immediate renal activity. May produce symptoms due to lodging of particles in the cerebral/coronary circulations.
3- Surgically absent lung (s/p pneumonectomy)
4- Patients with poor respiratory function:
Intubated ICU patient with cardiopulmonary instability.
5- Pediatric patients:
V/Q Scintigraphy in Pregnancy:
Characteristic Patterns:
Etiologies of V/Q Mismatch:
* Pulmonary Embolism (thrombotic, septic, air, etc.): Acute or Chronic. Note: Fat emboli typically produce a mottled appearance to the scan due to the presence of many small fat emboli.
* Pleural effusion/Atelectasis: More typically, atelectasis produces a ventilatory abnormality that demonstrates normal or minimally reduced perfusion.
* Pneumonia.
* Tumor/Hilar adenopathy: Bronchi are more resistant to extrinsic compression than are the pulmonary arteries because of their rigid cartilaginous rings.
* Vasculitis/Radiation Treatment: Can reduce regional lung perfusion. Radiation treatment results in obliteration of the microvasculature. Perfusion defects from radiation are usually geometric and follow the treatment port. They are typically non-segmental. Ventilation may also be reduced in the irradiated area, but it is usually less affected than perfusion.
* Pulmonary artery atresia or hypoplasia. Segmental or branch pulmonary artery stenosis.
* Fibrosing mediastinitis can lead to central vascular obstruction.
* AVM: Short circuits delivery of the particulate tracer to the regional pulmonary precapillary arterioles.
* CHF: Multiple non-segmental perfusion defects can be seen.
* Pulmonary artery sarcoma.
* Intravenous drug use: Can see bizarre perfusion patterns resulting from embolization of materials such as talc. Multiple small defects are most commonly seen, but larger defects may be noted and can occur in the absence of ventilatory abnormalities.
Etiologies of a heterogeneous perfusion pattern:
(Many small and medium sized defects scattered throughout both lungs.)
* CHF: May be characterized by diffuse or scattered non-segmental perfusion defects. Typically there is redistribution with reversal of the normal perfusion gradien; i.e.. upper lobes better perfused than lower lobes in an upright patient. A fissure sign refers to an oblique linear area of decreased perfusion due to pleural fluid tracking in the fissure.
* Lymphangitic carcinomatosis: Hematogenous microemboli which grow from the capillaries into the lymphatics. Contour Mapping in lymphangitic carcinomatosis appears as linear defects outlining the margins of the bronchopulmonary segments.
* Non-thrombogenic emboli: Fat, Septic, Amniotic Fluid.
* Vasculitis.
* Chronic Interstitial Lung Disease.
* Primary Pulmonary Hypertension [10]: A characteristic "mottled" perfusion pattern consisting of multiple non-segmental perfusion defects with a normal ventilation exam has been described in patients with primary pulmonary hypertension (PPH) [10]. The pattern is thought to be secondary to vasoconstrictive occlusion of small pulmonary arteries [10]. The degree of heterogeneity to the perfusion pattern correlates with the clinical severity of PPH [10].
Etiologies of decreased perfusion to one lung: [2,6]
Unilateral decreased perfusion (or predominantly decrease unilateral perfusion) can be
seen in 2% to 6% of V/Q scans [1,3].
* Pulmonary embolism: Thromboembolism as a cause of unilateral decreased pulmonary perfusion was previously felt to be uncommon. Unilateral decreased perfusion can be secondary to PE in up to 23% of cases [3]. Generally perfusion defects are noted in the opposite lung as well. However, chronic PE has been shown to be the etiology for unilateral hypoperfusion in up to 67% of cases [1].
* Pulmonary agenesis: There will also be absent ventilation and the CXR will show a small, opaque hemithorax.
* Hypoplastic lung (Pulmonary artery atresia): There is usually ventilation to a small lung which demonstrates no evidence of perfusion. On CXR the involved lung is usually small, hyperlucent, and contains few normal pulmonary markings [Miller, p.68].
* Swyer-James Syndrome: Characterized by bronchial destruction. Ventilation images with Xenon-133 reveal decreased wash-in and delayed clearance of the tracer on the involved side. Images acquired with Krypton-81m generally demonstrate absent ventilation. Perfusion will typically be decreased and inhomogeneous, but may be severely reduced and nearly inapparent. In general, however, the disorder produces a more severe impairment of ventilation than of perfusion in the affected lung.
* Pneumothorax
* Massive pleural effusion
* Tumor/Mediastinal mass: A central mass can compress or occlude the pulmonary artery resulting in absent perfusion. Endobronchial obstruction can produce hypoxic vasoconstriction.
* Pulmonary artery sarcoma
* Aortic dissection: Results in unilateral right lung absent perfusion due to direct compression of the right pulmonary artery by the intramural hemorrhage within the adjacent ascending aorta.
* Fibrosing mediastinitis: Vessels (soft walls) will be occluded by the progressive fibrosis prior to occlusion of the bronchi (cartilagenous walls)
* Shunt procedures for congenital heart disease
* Lung transplantation with non-perfusion of the native (diseased) lung
Artifacts on V/Q scintigraphy:
* "Hot Spots": Occur due to injection of blood clots which inadvertently formed in the syringe.
* Effusions: If the patient is scanned supine, effusions may collect posteriorly/superiorly and mimic a defect due to attenuation.
* Liver uptake on ventilation images: Xenon is fat soluble (and somewhat soluble in
blood) and it may be deposited in the liver- especially when there is fatty infiltration.
Increased RUQ activity should not be mistaken for RLL trapping.
REFERENCES:
(1) Radiology 1999; Bergin CJ, et al. Identifying the cause of unilateral hypoperfusion in
patients suspected to have chronic pulmonary thromboembolism: Diagnostic accuracy of
helical CT and conventional angiography. 213: 743-749
(2) AJR 1998; Pickhardt PJ, et al. Unilateral hypoperfusion or absent perfusion on pulmonary scinitgraphy: Differential diagnosis. 171: 145-150
(3) AJR 2001; Chow B, et al. Unilateral absence of pulmonary perfusion mimicking pulmonary embolism. 176: 712
(4) J Nucl Med 2001; Hartmann IJC, et al. Technegas versus 81mKr ventilation-perfusion scintigraphy: A comparitive study in patients with suspected acute pulmonary embolism. 42: 393-400
(5) J Nucl Med 1997; Mackey DW, et al. Physical properties and use of pertechnegas as a ventilation agent. 38: 163-167
(6) Semin Nucl Med 1995; Sutter CW, Stadalnik RC. Unilateral absence or near absence of pulmonary perfusion on lung scanning. 25: 72-74
(7) Radiology 1998; Boiselle PM, et al. Pulmonary embolism in pregnant patients: Survey of ventilation-perfusion imaging policies and practices. 207: 201-206
(8) Prog Cardiovasc Dis. 1994; Stein PD, Gottschalk A. Critical review of ventilation/perfusion lung scans in acute pulmonary embolism. 37: 13-24
(9) J Nucl Med 1996; Cabahug CJ, et al. Utility of technetium-99m-DTPA in determining regional ventilation. 37. 239-244
(10) J Nucl Med 2002; Fukuchi K, et al. Quantitative analysis of lung perfusion in patients with primary pulmonary hypertension. 43: 757-761