Transposition of the Great Vessels:
View cases of transposition of the great vessels
Clinical:
Transposition of the great vessels (TGA) is the most common
cyanotic heart disease to present at birth (within the first 24
hours) and is the second most common cyanotic congenital heart
disease diagnosed in the first year of life (following tetrology
of Fallot) [6]. It accounts for 5-7% of congenital cardiac
malformations and it is most common in infants of diabetic mothers
[3]. Males are affected more than females (2:1) [9]. The condition
is isolated in 90% of cases and it is rarely associated with any
specific syndrome, chromosomal abnormality, or an extracardiac
malformation [3].
It is defined by concordant atrioventricular and discordant
ventriculoarterial connection [2]. There are two parallel
circulations with impaired oxygenation of peripheral organs [9].
The condition can be tolerated in utero owing to oxygenated blood
from the umbilical vein flowing through the left atrium, LV, PA,
and patent ductus arteriosus to the aorta and the systemic
circulation [9]. After birth, the condition requires a
communication between the parallel pulmonary and systemic
circulations (most commonly a VSD (50%), ASD, or PDA) for patients
to survive [7,9].
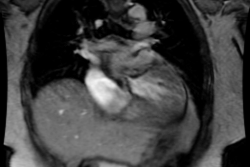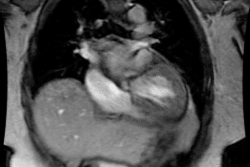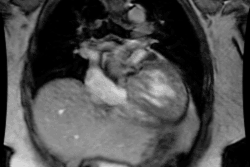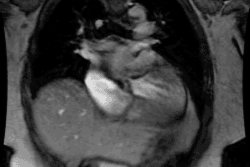
Patients present with intense, neonatal cyanosis, tachypnea, & CHF. Transposition means reversal of the normal anterior to posterior relationships and there are two variants- dextrotransposition (complete) and levotransposition (congenitally corrected) [6].
In D-Transposition (dextro-transposition):
- There is atrio-ventricular concordance- the RV is connected to the right atrium and the LV is connected to the left atrium.
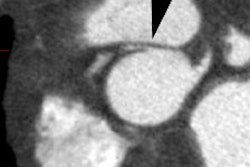
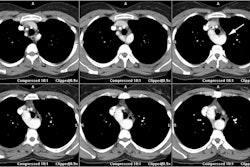
- Aorta arises from the RV (trabeculated ventricle) and supplies the systemic circulation. The aorta is anterior and is aligned with or slightly to the right of the pulmonary artery.
-- Pulmonary artery arises from the LV (smooth ventricle) and supplies the pulmonary circulation. The pulmonary artery lies posterior to the aorta.
D-transposition is summarized by the annotation S,D,D- in which S
stands for visceroatrial situs solitus (the normal morphologic
arrangement with the right atrium located to the right of the
morphologic LA, the IVC to the right or the aorta, the liver on
the right, and the spleen on the left); the first D stands for a
D-loop (location of the morphologic RV on the right side and the
morphologic LV on the left side); and the second D stands for
D-malposition of the aortic valve in relation to the pulmonic
valve- in this case the aortic valve is situated to the right of
and anterior to the pulmonary valve and the great arteries are
parallel instead of crossing as they do in a normal heart [6].
Associated findings:
VSD is the most common associated anomaly seen in 50% of cases
[9]. Left ventricular outflow tract obstruction (subpulmonic) is
seen in 30-33% of cases and is caused by deviation of the
infundibular septum in a posterior malalignment VSD, pulmonic
valve dysplasia, or a fibromuscular ring [9]. Other authors note
pulmonary atresia/stenosis in up to 20% of cases and these
patients will demonstrate decreased pulmonary blood flow.
A right arch is also found in 20% of cases. Coronary artery anomalies can also be seen in up to 33% of cases [9].
Surgical correction during infancy is the preferred treatment for TGA [9]. There are various forms of surgical treatment and more than 80% of neonates treated surgically survive to adolescence [6]. The Jatene operation is a definitive corrective "arterial" switch and this has generally replaced other surgical treatments becoming the treatment of choice as it offers a better long-term outcome [2,4,7,8,9]. The Jatene procedure is optimally performed in neonates within the 1st 1-2 weeks (first month [9]) of life and is associated with a 90% survival rate [5]. In the procedure, the aorta is transected above the sinus, the PA transected before its bifurcation, and coronary buttons are harvested with a patch of aorta [9]. The distal PA is brought anterior to the aorta (the LeCompte maneuver) and anastomosed to the remaining aortic root to form a neopulmonary artery [9]. The distal aorta is anastomosed to the posteriorly located proximal PA to form a neoaorta to which the coronary arteries are reimplanted [9]. Any associated septal defects are closed at the same time [9]. However, if a VSD closure is performed at the time of the arterial switch operation, surgical survival is somewhat less favorable [8].
Early surgical outcomes are excellent when the semilunar valves
are of equivalent size, the valve commisures are aligned, and the
coronary artery pattern is normal [8] Long term complications of
the procedure include coronary artery stenosis (due to suture line
fibrosis, kinking ,stretching, or compression by the pulmonary
arteries [6,9]. Coronary artery stenosis is the msot common cause
of early mortality after the arterial switch procedure with an
incidence of 2-11% [9]. Other complications include neoaortic root
dilatation with valve regurgitation [6]. Stenosis of the
supravalvular neopulmonary artery or branch pulmonary artery can
be seen in 5-40% of patients and intervention is required in up to
28% of patients [9].
Previous surgical treatments of d-TGA: The Mustard and Senning procedures involved the creation of an intra-atrial baffle to redirect flow entering the heart to the embryologic LA and subsequently, into the PA [5]. In the Mustard procedure the atrial septum is removed and replaced by a pericardial graft which directs pulmonary venous blood toward the right ventricle and systemic venous blood towards the left ventricle. This procedure is most successful in infants with an intact ventricular septum. The Senning procedure produces the same results, but a flap is made from the native atrial septum and sewn into the posterior wall of the LA [5,7]. In the Rashkind procedure a percutaneous balloon atrial septostomy is performed to permit better admixture of blood at the atrial level. In the Blalock-Hanlon procedure there is a surgical atrial septectomy.
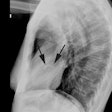
X-ray:
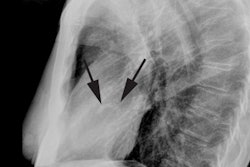
Classic plain film findings in transposition include shunt vascularity (which develops over the first few days due to closure of the ductus arteriosus). Note however, that the pulmonary vascularity may not be prominent if there is an associated pulmonic valve stenosis which is seen in 20% of cases. The heart is usually normal at birth and for the first few days, but becomes enlarged and develops an "egg on side" shape characterized by a narrow superior mediastinum which is due to a stress induced thymic atrophy and the abnormal relationship of the great vessels as the aorta and pulmonary artery are aligned front to back [3]. There is an absent pulmonary artery segment and an absent aortic knob. Asymmetric pulmonary blood flow greater to the right lung may be seen.
REFERENCES:
(1) Pediactric Clinics of North America 1999;
Grifka RG. Cyanotic congential heart disease with increased
pulmonary blood flow. 46(2): 405-425
(2) Radiographics 2007; Leschka S, et al. Pre- and postoperative evaluation of congenital heart disease in children and adults with 64-section CT. 27: 829-846
(3) Radiographics 2007; Ferguson EC, et al. Classic imaging signs of congenital cardiovascular abnormalities. 27: 1323-1334
(4) AJR 2008; Takaki MTT, et al. Nonatherosclerotic cardiovascular findings on MDCT coronary angiography: a selection of abnormalities. 190: 934-946
(5) Radiology 2008; Gaca AM, et al. Repair of congenital heart disease: a primer-part 1. 247: 617-631
(6) AJR 2009; Wiant A, et al. CT evaluation of congenital heart disease in adults. 193: 388-396
(7) Radiographics 2010; Frank L, et al. Cardiovascular MR imaging
of conotruncal anomalies. 30: 1069-1094
(8) J Cardiovasc Comput Tomogr 2013; Han BK, Lesser J. CT imaging
in congenital heart disease: an approach to imaging and
interpreting complex lesions after surgical intervention for
tetrology of Fallot, transposition of the great arteries, and
single ventricle. 7: 338-353
(9) Radiographics 2021; Canan A, et al. Multimodality imaging of
transposition of the great arteries. 41: 338-360