Takotsubo cardiomyopathy or Transient Left Ventricular Apical Ballooning Syndrome:
Clinical:
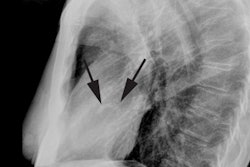
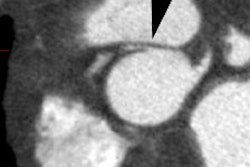
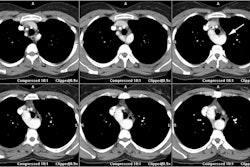
Transient LV apical ballooning syndrome is a rare form of transient LV dysfunction that mimics an acute coronary syndrome/acute MI [1,3]. The name Takotsubo is related to the morphology of the left ventricle, which shows a resemblance to a Japanese round-bottomed narrow-necked fishing pot used for trapping octopus [4]. Patients present with anginal, substernal chest pain, ST segment elevations on ECG (in the precordial leads in up to 68% of patients [3]), diffuse T-wave inversions (up to 97% of cases [3]), and often mild elevated cardiac enzymes (86% of patients have mildly elevated Troponin levels and 74% have slightly elevated creatine kinase-MB levels) [2,3]. In contrast to the pattern associated with acute MI, the cardiac enzymes in Takotsubo cardiomyopathy (TC) peak at presentation and quickly normalize [3]. The disorder is characterized by extensive regional wall motion abnormalities with hypokinesis or dyskinesis of the apical segments and hypercontractility of the basal segments in the absence of atherosclerotic disease at catheterization (significant CAD is found in only 5% of patients at angiography) [1,2,3]. The acute phase of the disorder is felt to be related to metabolic dysfunction at the cellular level [1]. Formal diagnostic criteria have not yet been established, but the following have been suggested: 1- transient LV wall motion abnormalities involving the apical and/or midventricular myocardial segments with wall motion abnormalities extending beyond a single epicardial coronary distribution; 2- absence of obstructive CAD; and 3- new ECG abnormalities such as transient ST-segment elevation and/or diffuse T-wave inversions or Troponin T evelation [4].
The disorder is most frequently seen in post menopausal women- 87% of reported cases (average age 62-75; mean age 68 years), often in the setting of acute emotional or physiologic stress [2,3,4]. Although the exact pathophysiology of the disorder is unknown, there is evidence that excessive sympathetic stimulation is integral to the development of TC (physical or emotional stress is the precipitating factor in about 80% of cases) and neurogenic myocardial stunning has been demonstrated in patients using I-123 MIBG scintigraphy [3,4]. Stress induced coronary epicardial spasm or endothelial dysfunction resulting in myocardial stunning is another possible etiology for the condition [4].
Most patients fully recover within a few weeks (in-hospital mortality is less than 1%) [2,3]. However, patients can develop severe LV dysfunction requiring supportive measures- mid and distal LV akinesia with compensatory basal hyperkinesia can cause LV cavity obliteration that results in LV outflow obstruction and intraventricular pressure gradients [3]. There is evidence that dobutamine is contraindicated in patients with TC, especially in the presence of outflow obstruction and that beta blockade may be beneficial in the treatment of the disorder [3]. The recurrence of the disorder is rare (estimated at 2.9%/year) [4].
A variant presenting as basal ballooning (that spares the mid-LV region and has paradoxical apical hyperkinesis) has recently been described in young pre-menopausal women [3].
X-ray:
Stress myocardial perfusion imaging during the acute phase will demonstrate a reversible perfusion defect in the apex and reduced apical coronary flow reserve [1,4]. Metabolic imaging with FDG has been reported to demonstrate a severe reduction in glucose metabolism in the apical segments (during hyperinsulinemic-euglycemic clamp imaging) [1]. A flow-metabolism mis-match pattern has been described with relatively preserved or moderately reduced myocardial perfusion to the apex, but markedly abnormal decreased metabolism (with either BMIPP or FDG) [3,5]. A reversible impairment of coronary flow reserve in the apical myocardial segements using quamtitative perfusion PET has been demonstrated suggesting that microcirculation dysfunction plays a key role in this disorder [5]. In general, the regional reduction of perfusion and impairment in glucose metabolism have a tendency to resolve completely within 3 months from presentation [4].
Regional denervation has also been reported in the apex during the acute phase on I123 MIBG imaging [1,5]. Normalization of FDG uptake with persistent decreased apical I123 MIBG uptake can be seen even at one year followup [5].
On MR, there is characteristic apical ballooning with akinesis or severe hypokinesis, while the basal portions of the LV demonstrate hyperkinesis [2]. Delayed contrast enhanced MR imaging does NOT show any evidence of delayed enhancement on post gadolinium imaging [1]. The apical abnormality resolves and normal function and imaging exams can be seen by 3 months following the event [1].
REFERENCES:
(1) J Nucl Cardiol 2008; Feola M, et al. Reversible impairment of coronary flow reserve in takotsubo cardiomyopathy: a myocardial PET study. 15: 811-817
(2) Radiographics 2009; Cummings KW, et al. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. 29: 89-103
(3) J Nucl Cardiol 2009; Dorfman TA, Iskandrian AE. Takotsubo cardiomyopathy: state-of-the-art review. 16: 122-134
(4) J Nucl Cardiol 2009; Verberne HJ, et al. Persisting myocardial sympathetic dysfunction in takotsubo cardiomyopathy. 16: 321-324
(5) J Nucl Cardiol 2009; Galiuto L, et al. Non-invasive imaging of microvascular damage. 16: 811-831