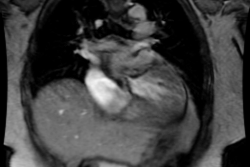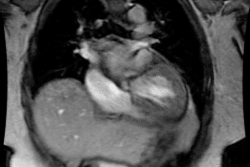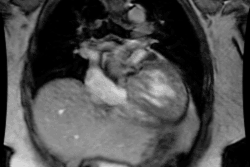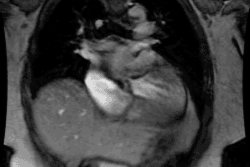
Atrial Fibrillation:
Clinical:
(See also pulmonary
venous anatomy)
Arrhythmogenic foci originating within the pulmonary veins are
important causes of both paroxysmal and persistent atrial
fibrillation [1]. In most individuals, sleeves of left atrial
myocardium extend into the pulmonary veins for a distance of 2-17
mm [1]. In up to 94% of patients, atrial fibrillation is triggered
by ectopic electrical activity from these sleeves of tissue [5].
These sleeves are longest in the superior pulmonary veins and
thickest at the venoatrial junction of the left superior pulmonary
vein [1]. Hence, almost 50% of the foci arise in the left superior
pulmonary vein [1]. Other authors note the sleeves are thickest at
the inferior walls of the superior veins and the superior walls of
the inferior veins [6].
Other sites of ectopic electrical foci have also been identified
and include the LA wall accessory appendages and diverticula
(found in 10-46% of patients and most commonly located in the
right anterior-superior wall of the LA) [5]. LA diverticula
contain normal myocardial wall structure and contract in synchrony
with the rest of the atrium [5]. Accessory appendages are
characterized by the presence of trabeculated myocardiukm withy
the same wall structure as the surrounding myocardium and have
also been shown to have significant contractile properties [5].
Transvenous radiofrequency ablation of these foci is performed to
electrically disconnect them from the left atrium [1]. Larger
pulmonary vein size has been reported to be an independent
predictor of recurrent atrial fibrillation after ablation [6].
Complications (reported rate 3.9-5%) from transcatheter RF
ablation include stroke, cardiac tamponade, vascular injury,
hemothorax, heart block, mitral valve injury, phrenic nerve
injury, and gastroparesis [4]. An increased risk for complications
is associated with older age (>70 years) and female gender [4].
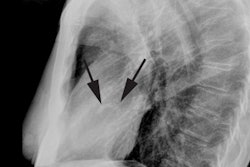
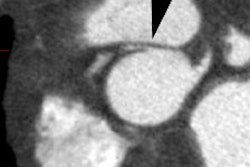
Pulmonary vein scarring and stenosis (3-8% of patients) can occur
[4,6]. Patients can present with variable symptoms- chest
discomfort, hemoptysis, and shortness of breath [4]. Pulmonary
vein stenosis is usually treated with angioplasty or stent
placement (although angioplasty alone has a high failure rate and
restenosis) [6].
Gastroparesis has been reported in under 1% of cases and occurs
secondary to vagal nerve injury that supplies the pyloric
sphincter and gastric antrum [4]. Th vagus nerve fibers are
located over the anterior and posterior aspects of the esophagus
and the anterior fibers lie in close proximity to the left atrium
and veno-atrial junction [4]. Approaches to prevent vagal injury
include reduction in the RF energy, shorter duration of energy
delivery, and limiting ablation sites using elctro-anatomic
mapping [4]. Most cases of gastroparesis will gradually improve
over time [4].
X-ray:
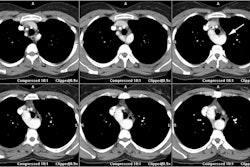
MDCT can be performed to evaluate for pulmonary venous anomalies and to define ostial orientation and distance from each ostium to the bifurcation of each pulmonary vein [1,2].
REFERENCES:
(1) AJR 2004; Cronin P, et al. MDCT of the left atrium and pulmonary veins in planning radiofrequency ablation for atrial fibrillation: a how-to guide. 183: 767-778
(2) Radiology 2005; Jongbloed MRM, et al. Atrial fibrillation:
multi-detector row C of pulmonary vein anatomy prior to
radiofrequency catheter ablation- initial experience. 234: 702-709
(3) Radiographics 2011; Aguilera AL, et al. Radiography of
cardiac conduction devices: a comprehensive review. 31: 1669-1682
(4) J Nucl Cardiol 2011; Vallet W, Unger SA. The role of nuclear
medicine in diagnosing complications related to catheter-based AF
ablation. 18: 1103-1106
(5) J Cardiovasc Comput Tomogr 2012; Lazoura O, et al. Prevalence
of left atrial anatomical abnormalities in patients with recurrent
atrial fibrillation compared with patients in sinus rhythm using
mutli-slice CT. 6: 268-273
(6) Radiographics 2017; Hassani C, Saremi F. Comprehensive
cross-sectional imaging of the pulmonary veins. 37: 1928-1954