Lymphangioleiomyomatosis:
View
images
of
lymphangioleiomyomatosis
Clinical:
Lymphangioleiomyomatosis is characterized by a progressive proliferation of spindle cells which resemble immature smooth muscle in the lung parenchyma and along lymphatic channels which dilate. Proliferation of spindle cells along the bronchioles leads to air trapping and the formation of thin walled cysts. The disorder progresses to end-stage lung disease secondary to post-obstructive emphysema. The disorder occurs only in females of child bearing age (17-50y) and is characterized by progressive lung disease with increasing dyspnea on exertion (presenting symptom in 59% of patients), cough (39% at presentation), chylous effusion in 10-20% (also chylous ascites), and spontaneous pneumothorax has been reported in 39-53% of patients at presentation and in up to 81% during the course of the disease [6]. Recurrent pneumothorax is common with conservative treatment [6]. Pulmonary hemorrhage occurs in 8-15% of patients [5]. Spirometry studies demonstrate chronic airway obstruction with increased lung volume, decreased FEV1, decreased FEV1/FVC ratio, and decreased CO2 diffusion [5]. The diagnosis is often delayed with an average of 44 months from onset of symptoms until diagnosis [6].
Extrapulmonary manifestations are commonly found. Renal angiomyolipomas are the most common intraabdominal manifestation of the disorder and can be found in 20% to 54% of patients with LAM [2,3,5,6]. There is no increased risk for renal cell carcinoma in LAM patients [3]. Angiomyolipomas seen in patients with lymphangioleiomyomatosis may be related to perivascular epitheliod cell tumors (PEComas) that result from the dysregulated mammalian target of rapamycin [12]. Other extrapulmonary manifestations include enlarged abdominal lymph nodes (up to 40% of patients [5]), lymphangiomyoma (cystic retroperitoneal mass found in up to 20% of patients [5], chylous ascites (10-30%), enlarged throacic duct (9%), and hepatic angiomyolipomas (4%) [3]. Abdominal lymphadenopathy occurs because of lymphatic infiltration (lymph nodes are usually found to be replaced with smooth muscle) and can be identified in up to 39% of patients [3,11]. The enlarged nodes can measure up to 4 cm in size [3] and are referred to as lymphangioleiomyomas [11]. The presence of abdominal/retroperitoneal adenopathy correlates with the severity of lung disease [3]. The nodes have been shown to exhibit a diurnal variation in size- enlarging during the day and shrinking at night [11].
The disorder is believed to be hormonally mediated and menstruation, pregnancy, and exogenous estrogen treatment (including oral contraceptives) may exacerbate the disorder [6]. Therapy has been direct towards reducing circulating estrogen levels with medroxyprogesterone [8]. However, the efficacy of the therapy is controversial and even with medical treatment, survival is between 40% to 78% at 8.5 years [2,6].
Bilateral lung transplantation is another treatment option for patients with LAM. Indications for transplant include progression despite medical treatment, and FEV1/forced vital capacity ratio of less than 50%, total lung capacity of greater than 103%, and an FEV1 of less than 30% [5]. Unfortunately, patients with LAM are at greater risk for morbidity and mortality from transplant due to pleural adhesions (which can lead to significant operative blood loss), native lung pneumothorax, and chylous pleural effusion [2,4]. LAM can recur in the transplanted lung in about 10% of patients [2,4].
Tuberous sclerosis:
Tuberous sclerosis (TS) occurs as a result of a gene mutation (TSC1 on chromosome 9 or TSC2 on chromosome 16) and the disorder can be sporadic (60% of cases) or inherited [7,13]. Adenoma sebaceum occurs in 75% of patients and is characterized by pink and red papillary lesions on the face, particularly the cheeks, malar eminences, and nose [13]. Other cutaneous manifestations include periungual fibromas (about 25% of patients) and Fitzpatrick patches (ash-leaf spots- hypomelanotic patches) [13]. Patients with tuberous sclerosis can also develop lymphangioleiomyomatosis. Pulmonary disease is thought to be rare (about 2.3% of TS patients), but clinically silent involvement likely occurs in a larger percentage [6]. Globally, lymphangioleiomyomatosis associated with tuberous sclerosis is 5- to 10 fold more common than sporadic lymphangioleiomyomatosis [12]. Up to 35% of women with TS have lung cysts that are identical histologically to LAM (lung cysts are rarely seen in men with the condition [13]) [10]. Compared to patients with primary LAM, pulmonary involvement tends to be less extensive in TS patients [7]. Also- thoracic duct dilatation, chylous effusion and ascites are also less common [7]. However, patients with tuberous sclerosis tend to have a higher incidence of renal AML's (93% versus 32%) and hepatic AML's (33% versus 2%) [7]. Renal AML's tend to be multiple and bilateral in TS patients, while they are unilateral and single in LAM patients [7]. TS patients also have a higher frequency of non-calcified lung nodules (12% versus 1%) which are felt to be due to multifocal micronodular pneumocyte hyperplasia [7]. Another feature that aids in identification of TS patients is the presence of 4 or more sclerotic bone lesions (histologically these lesions resemble bone islands) [10].
Recently, the presence of well-circumscribed foci of fat within the myocardium of TS patients has also been described (50-64% of patients) [9,14]. The fatty foci are most commonly located within the interventricular septum and the wall of the left ventricle [9].
X-ray:
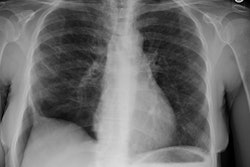
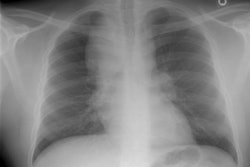
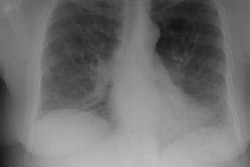
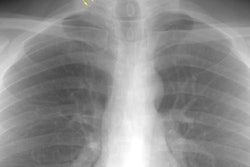
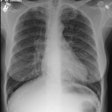
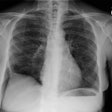
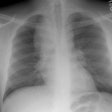
CXR: On CXR the disorder is characterized by bibasilar coarse reticular lung disease with normal to hyperinflated lungs (large volumes are seen in about 22-50% of cases [6]). The reticular appearance is due to the summation of multiple thin-walled cysts and is seen in about 66-90% of patients [6]. A chylous pleural effusion is noted in 10-20% of cases (but can be seen in up to 44% of patients [6]), and pneumothorax in 40-50% [6]. The end-stage of the disorder produces a honeycomb lung.
Computed tomography: On HRCT there are characteristically numerous thin walled cysts which are surrounded by relatively normal lung parenchyma. The majority of the cysts measure between 2-5 mm in size, but larger cysts between 6-30 mm are also seen [6]. The cysts ar thought to arise from air trapping associated with the peribronchovascular proliferation [12]. The cysts tend to enlarge as the disease progresses, are distributed diffusely throughout the lungs, and are usually round or ovoid (irregular shapes are uncommon, a distinction from eosinophilic granuloma) [12]. A slight increase in linear interstitial markings, interlobular septal thickening, or patchy areas of ground-glass opacification are also be seen, but findings of significant fibrosis are usually absent. Hilar and mediastinal adenopathy has been reported in up to 50% of patients [6].
REFERENCES:
(1) Radiol Clin North Am 1994 Jul;32(4):745-757
(2) Radiology 1999; Collins J, et al. Lung transplantation for lymphangioleiomyomatosis: Role of imaging in the assessment of complications related to the underlying disease. 210: 325-332
(3) Radiology 2000; Avila NA, et al. Lymphangioleiomyomatosis: Abdominopelvic CT and US findings. 216: 147-153
(4) Radiology2001; Collins J, et al. Frequency and CT findings of recurrent disease after lung transplantation. 219: 503-509
(5) Radiographics 2002; Pallisa E, et al. Lymphangioleiomyomatosis: pulmonary and abdominal findings with pathologic correlation. 22: S185-198
(6) Radiographics 2005; Abbott GF, et al. Lymphangioleiomyomatosis: radiologic-pathologic correlation. 25: 803-828
(7) Radiology 2007; Avila NA, et al. Sporadic lymphangioleiomyomatosis and tuberous sclerosis complex with lymphangioleiomyomatosis: comparison of CT features. 242: 277-285
(8) Radiology 2007; Attili AK, Kazerooni EA. Case 116: Lymphangioleiomyomatosis. 244: 303-308
(9) Radiology 2009; Adriaensen ME, et al. Fatty foci in the myocardium in patients with tuberous sclerosis complex: common finding on CT. 253: 359-363
(10) Radiology 2010; Avila NA, et al. CT of sclerotic bone lesions: imaging features differentiating tuberous sclerosis complex with lymphangioleiomyomatosis from sporadic lymphangioleiomyomatosis. 254: 851-857
(11) AJR 2011; Avila NA, et al. Imaging
features of
lymphangioleiomyomatosis: diagnostic
pitfalls. 196:
982-986
(12) AJR 2011; Seaman DM, et al. Diffuse cystic lung disease at
high-resolution CT. 196: 1305-1311
(13) Radiographics 2011; Kanne JP, et al. Beyond skin deep:
thoracic
manifestations of systemic disorders affecting the skin. 31:
1651-1668
(14) Radiology 2015; Tresoldi S, et al. Myocardial fatty foci in adult patients with tuberous sclerosis complex: association with gene mutation and multiorgan involvement. 277: 398-405