Aortic Aneurysm:
View images of aortic aneurysm
Clinical:
The aortic annulus is a virtual ring surrounding the
ventriculo-aortic junction just below the lowest insertion
points of the aortic valve [39]. The sinuses of valsalva are the
outward bulge of the aortic root associated with each of the
three aortic valve cusps [39]. The aortic root is defined as the
part of the ascending aorta that contains the aortic valve,
annulus, and sinuses- this is the region from the
ventriculoaortic junction (aortic valve ring) to the sinotubular
junction [17,27]. The sinotubular junction is the slight
waist-like narrowing between the aortic root and the tubular
portion of the ascending aorta [39]. The tubular portion of the
ascending aorta begins above the sinuses of valsalva, at the
sinotubular junction and extends to the origin of the right innominate (brachiocephalic)
artery [17,19]. The aortic arch
extends from the right brachiocephalic
artery to the attachement of the ligamentum arteriosum
[17]. From the ligamentum arteriosum to the diaphragmatic hiatus
is the decending thoracic aorta
[17].
Aortic aneurysms can be classified as either fusiform or saccular
(a focal out-pouching of the wall). An aortic aneurysm has been
defined as segmental dilatation of ≥ 50% of the full
thickness of the vessel involving any segment from the aortic
root to the abdomninal aorta [43]. The normal diameter of the
mid-ascending aorta should always be less than 4 cm, and that of
the descending aorta no more than 3 cm [17]. Thoracic aortic
measurements greater than 4 cm are considered consistent with an
aeurysm [39]. The risk of rupture increases with the size of the
aneurysm [17,19]. The reported risk
is 0% if less than 4 cm, 16% for aneurysms between 4 to 5.9 cm,
and 31% if 6 cm or more [43]. The risk for rupture when the
aneurysm is greater than 5.5 cm in diameter is generally
perceived to exceed the risk of operation [39].
Although thoracic aortic aneurysms expand at a slower rate than abdominal aortic aneurysms, surgical repair is contemplated when the aneurysm reaches a diameter of 5.5 cm (and for descending thoracic aneurysms greater than 6.5 cm) [17,28]. An annual growth rate of greater than 1cm [17] or a diameter greater than 4.5 cm with an increase of at least 0.5 cm in the preceding 6 months are other accepted indications for surgical repair [17,19]. Although, other authors indicate that surgical repeair is recommended in patients with an aneurysm that grows more than 0.5 cm in one year [28]. Rapid aneurysm growth (more than 0.5-1 cm per year) is also a consideration for elective repair, even if the absolute size criteria has not been met [39]. Earlier intervention is recommended in patients with Marfans syndrome (between 4.5-5 cm) or bicuspid aortic valves (more than 4 cm due to risk of dissection) [17,28,39].
In symptomatic patients, aneurysms are repaired regardless of
size [28].
Marfan syndrome:
Marfan syndrome is an autosomal dominant connective tissue
disorder caused by mutations in the gene (FBN1) that encodes
fibrillin-1 [39]. Approximately 25% of cases result from a
sporadic mutation of the FBN1 gene [39]. Classically, Marfan
syndrome results in a tulip-shaped configuration to the aortic
root (annuloaortic ectasia) with dilatation of the aortic
annulus and sinuses of valsalva, and effacement of the
sinotubular junction [39]. Dilatation of the aortic annulus
results in aortic insufficiency in 15-44% of patients [39].
Ehlers-Danlos syndrome, vascular type:
Ehlers-Danlos syndrome, vascular type (Type IV) is a rare autosomal dominant disorder that results from mutations in the gene (COL3A1) that encodes type III procollagen [39]. Excessive tissue fragility predisposes patients to vascular complications including aortic aneurysm, dissection, and rupture [39].
Surgical repair:
Prior to surgical repair of ascending thoracic aortic aneurysms it is important to document if the aneurysm involves the aortic arch or extends to the inominant artery. In such cases hypothermic circulatory arrest will be necessary for the procedure. This entails cooling the patient systemically to below 19 degrees Celsius, placing the patient in the Trandelenburg position, and stopping the cardiopulmonary bypass circuit. Hypothermic circulatory arrest increases the complexity, length, and mortality associated with surgery. It is also used for some patients with severe aortic calcification where clamp placement leads to an increased risk for stroke due to thromboembolic phenomena, in patients with fragile aortic tissue (Marfan's) where clamping may result in aortic laceration, and in patients undergoing repeat aortic surgery [1].
The mortality rate for elective surgical repair of thoracic
aortic aneurysms can be as high as 7-12% [15]. The two most
common techniques for surgical repair are interposition graft
and inclusion graft [17]. After the affected segment has been
excised, an interposition graft is sewn end-to-end and vascular
branches such as the coronary arteries are reimplanted
[17]. An inclusion graft is inserted into the aortic lumen and
the native aorta is wrapped around the synthetic graft, leaving
a potential space between the native aorta and the graft that
may thrombose (or even show
persistent blood flow that would not require intervention in the
absence of hemodynamic instability) [17,28]. Inclusion grafts
are not commonly performed because of improved surgical
techniques with interposition grafts [28]. The synthetic grafts
are most commonly composed of polyethylene (Dacron) and are
slightly hyperattenuating relative to the native aortic wall on
non-contrast imaging, but appear hypoattenuating relative to the
contrast opacified aortic lumen [28].
A supracoronary grafting is indicated in patients with an
ascending aortic aneurysm or atherosclerotic origin and normal
sinuses of Valsalva [28]. With this technique, the cononary
ostia are preserved, which minimizes the risk for
pseudoaneurysm, stenosis, thrombosis, and kicking at the
cononary anastamosis site [28]. Simultaneous placement of a
supracononary aortic graft and an aortic valve is known as the
Wheat procedure [28]. One complication associated with
supracoronary grafting is the develoment of dissection or
aneurysm of the native aorta proximal to the graft- particularly
in patients with Marfan syndrome or annuloaortic ectasia [28].
The Bentall procedure is performed for patients with both
aortic valvular disease and dilatation of the sinuses of
valsalva whose aortic root walls are too vulnerable to allow
suturing of the proximal end of the aortic prosthesis [28]. In
the procedure, the native aortic root and aortic valve are
replaced with a composite graft that consists of both ascending
aorta and aortic valve grafts- the cononary arteries are then
anastamosed to the graft [28]. Because of the risk of
pseudoaneurysm formation at the conronary anastamosis, a
modified Bentall procedure (button Bentall or Carrel patch) was
dveloped in which a "button" of of the aorta encircling the
coronary ostia is removed with the coronary artery, facilitating
implantation of the coronary artery to the graft [28]. A
potential complication of the Bentall and modified procedures is
formation of a pseudoaneurysm at the distal aortic anastamosis
[28].
The Cabrol procedure is an alternative to the modified Bentall
procedure in patients with aortic dissection, annuloaortic
ectasia, or atherosclerotic aneurysm in whom the button Bentall
cannot be performed due to severe atherosclerosis of the
ascending aorta (which precludes good-quality buttons) or severe
proximal coronary artery disease [28]. In this procedure, a
composite aortic root and aortic valve graft and a prosthetic
conduit anastamose the cononary ostia to the aortic graft in a
side-to-side manner [28]. The normal post operative appearance
of a rtroaortic conduit may mimick that of an intimal flap
related to a dissection [28]. Complications of the Cabrol
procedure include anastomotic leak, cononary graft insufficiency
from kinking or intimal hyperplasia, acute graft thrombosis, and
endocarditis [28].
Biologic grafts are used for repair in the Ross procedure [28].
The Ross procedure is performed in young patients with a dilated
aortic root and an aortic valve condition [28]. In the
procedure, the native aortic root and aortic valve are replaced
with the patient's own pulmonary valve and proimxal pulmonary
artery [28]. A synthetic or biologic pulmonary graft is then
performed [28]. Advantages of the Ross procedure include
improved hemodynamics, a lower risk for endocarditis, lower
thrombogenicity and decreased need for anticoagulation, and an
allowance for growth potential in children [28]. The most
frequently reported complication is aneurysmal dilatation of the
aortic root, but aortic dissection and pseudoaneurysm at the
proximal or distal anastomotic sites has also been described
[28].
Anortic valve-sparing procedure is indicated in patients with
an aneurysm at the level of the sinuses of Valsalva, with or
without coexisting aortic valvular insufficiency, and in
patients with aneurysms that invovle the sinotubular junction,
but with essentially normal aortic valve leaflets [28]. In the
procedure, the aneurysmal aortic is removed to the level of the
annulus, with the aortic valve left intact and the sinuses of
Valsalva reconstructed with a Dacron graft [28]. Because the
native valve is left intact, there is no need for long-term
anticoagulation therapy [28]. The procedure is especially
attractive in Marfan syndrome patients [28]. Aortic
insufficiency is the most frequent cited complication of
valve-sparing procedures [28].
Aneurysms or dissections that involve the aortic arch are
repaired with the elephant trunk procedure [28]. The elephant
trunk procedure consists of two stages [28]. In the first stage,
the ascending aorta and aortic arch are removed and replaced
with a graft that is inserted into the descending aorta where it
is left unattached and floats freely [28]. The great vessels are
anastomosed to the graft [28]. In the second stage, a second
graft replaces the descending aorta and is anastomsed to the
original graft [28]. Alternatively, fixation of the original
distal portion of the graft can be achieved by deploying an
endograft- a hybrid elephant trunck procedure [28]. Spinal cord
ischemia and paraplegia are potential complications of the
procedure [28].
Aortic root surgical complications:
Aortic root pseudoaneurysm: Aortic root pseudoaneurysm is a
rare complication (less than 0.5% of patients) [30].
Mediastinitis and graft infection are the most common risk
factors for the formation of a postoperative aortic root
pseudoaneurysm [30]. Other risk factors include underlying
aortic wall disease (Marfan syndrome), dissection of the native
aorta, and excessive use of biologic glue [30]. The most common
location is at the graft anasatomsis site, followed by the
coronary artery anastomosis site, aortotomy site, aortic
cannulation site, and needle vent site [30]. Many patients
present with acute symptoms such as chest pain, heart failure,
and sepsis, but some patients can be relatively asymptomatic
[30]. The lesion carries a high risk for rupture [30].
Coronary ostial aneurysm: A coronary ostial aneurysm can
develop at the coronary artery reimplantation site- especially
in patients with underlying connective tissue disorders such as
Marfan syndrome or Loeys-Dietz syndrome (a recently described
connective tissue disorder) [30]. Coronary ostial aneurysms can
develop in up to 43% of patients with Marfan syndrome and are
thought to devlop as a result of perioperative stretch of the
weakened coronary ostial wall [30].
Mediastinitis/graft infection: The incidence of mediastinitis
following cardiac surgery is reported to be between 0.4-5% [30].
A small amount of fluid or gas may be seen for several days or
weeks after removal of mediastinal drains [30]. The presence of
abnormally large amounts of low-attenuation material surrounding
the aortic graft or increasing fluid and soft tissue
infiltration on serial scans should raise the suspicion for
mediastinitis [30]. Persistent (longer than 6 weeks), new, or
increasing perigraft air may indicate infection with a
gas-producing organism or a fistula with the adjacent bronchus
or esophagus [28].
Sternal dehiscence: Sternal dehiswcence and sternal wound
infection are serious complications that are seen in 1-7% of
patients who have undergone cardiac surgery [30]. Risk factors
include obesity, lung disease, diabetes, history of prior chest
wall radiaiton, renal disease, steroid use, and reoperation
[30]. Sternal dehiscence may occur alone or in association with
mediastinitis [30]. CT findings include displacement of sternal
wires, sternal erosion, or a cleft/sepration of the sternotomy
site [30].
Perigraft seroma: A perigraft seroma is a late complication of
polytetrafluoroethylene and polyester fiber grafts [30]. The
pathogenesis involves both failure of graft incorporation into
the native vessel wall and increased graft porosity [30]. Fluid
between the open aortic graft and the sac wall is a normal
finding on imaging in the period immediately following surgery
[30]. However, after 3 months any perigraft hematoma or fluid
should have resolved [30].
Etiologies for thoracic aortic aneurysms include:
1- Atherosclerotic vascular disease:
Atherosclerosis is the cause of about 70% of all thoracic
aortic aneurysms [17]. Atherosclerotic aneurysms can involve any
portion of the thoracic aorta, but most commonly descending
thoracic aorta [17] and typically produce a fusiform enlargement involving a long
segment of the vessel. About 28% of patients with thoracic
aortic aneurysms will also have an AAA [17]. Atherosclerotic
aneurysms of the ascending aorta typically spare the sinotubular junction and aortic valve
function until late in the disease process. Most thoracic aortic
aneurysms are asymptomatic. When symptoms do occur, they are
typically related to rupture, dissection, or compression of
adjacent structures. The treatment for descending thoracic
aortic aneurysms is surgical resection and replacement with
prosthetic graft. Mortality rates of up to 50% have been
reported in cases of emergent repair, and about 12% in elective
cases. Paraplegia occurs in 5-10% of patients. Translumenal endovascular stent grafting
offers an alternative method of therapy. The aneurysm must be at
least 2-3 cm from the origin of the left subclavian
artery to ensure that the stent does not cover the orifice of
this vessel. To limit exclusion of intercostal
arteries the length should be kept to a minimum. Mortality from
the procedure has been reported to be about 15%, and paraplegia
occurs in 4% of patients [3].
A hyperattenuating cresentic rim if seen on CT of a large abdominal aortic aneurysm is a specific sign of impending rupture [18].
On MR spin echo images, the wall of the vessel is thickened and irregular secondary to the presence of sclerotic plaque. Areas of signal void correspond to wall calcifications. Thrombus adherent to the wall of the aneurysm may be difficult to distinguish from plaque, although thrombus typically has a smooth interface with the vessel lumen.
2- Marfan's
disease / Ehlers-Danlos:
Marfan's syndrome is inhereted as an autosomal
dominant disorder with high penetrance,
but expression is highly variable. The disorder has been linked
to a fibrillin gene defect on
chromosome 15 (FBN1 gene mutation that encodes for fibrillin-1)
[4,33]. Marfan syndrome is a multisystem disorder that affects
the cardiovascular, ocular, and skeletal systems [33]. The major
manifestations include ectopia lentis, aortic root aneurysm or
dissection, and dural ectasia [33]. Surgical intervention is
indicated when the aortic root dimeter exceeds 5 cm, or when the
aneurysm growth exceeds 1 cm/year [33].
Ehlers-Danlos syndrome (EDS) is a group of clinically and
genetically heterogeneous heritable connective tissue disorders
[26]. There are six types of EDS currently recognized [26]. The
vascular type is a rare, autosomal-dominant disorder resulting
from a mutation in the COL3A1 gene encoding for type III
procollagen synthesis [26]. The more common vascular
complications with EDS include aortic aneurysm (but without
predilection for the aortic root), dissection, or rupture of
medium-size abdominal arteries (iliac, renal, and mesenteric)
[33]. Excessive tissue fragility predisposes patients with
vascular EDS to premature arterial, intestinal, or uterine
rupture during labor [26]. Patients also have thin ntranslucent
skin and a characteristic facial appearance (thin pinched nose,
thin lips, tight skin, hollow cheeks, and prominent staring eyes
due to decreased subcutaneous adipose tissue) [33].
Complications are rare during childhood, but more than 80% of
patients will have at least one complication by the age of 40
years [26]. Because of the risk of dissection, conventional
angiography is generally contraindicated in EDS patients [33].
Both Marfan's and Ehlers-Danlos are associated with cystic medial
necrosis that results in weakening of the aortic wall typically
resulting in aneurysms of the ascending aorta. Annuloaortic ectasia
is characterized by dilated sinuses of Valsalva
with effacement of the sinotubular
junction producing a pear-shaped aorta that tapers to a normal
aortic arch and the condition is most commonly associated with Marfans syndrome [17]. Associated valvular dysfunction is also common
secondary to dilatation of the aortic root. Other causes of annuloaortic ectasia
include idiopathic (about 1/3'd of cases), homocystinuria,
and osteogenesis imperfecta [17].Endovascular
stent-grafts are generally not recommended in the treatment of
aortic aneurysm in patients with connective tissue diseases
because they have a higher risk of endoleaks, reinterventions,
and disease progression compared to the general population [33].
On MR imaging, there is dilatation of the aortic root associated with complete effacement of the sinotubular junction (remember, this is not seen in atherosclerotic aneurysms until late). When viewed in an oblique coronal section this produces an "onion bulb" or "pear-shaped" appearance to the aortic root and this is considered to be characteristic of the disorders. Cine gradient images can be used to demonstrate regurgitant flow associated with valvular dysfunction.
3- Aortitis:
a) Non-infectious Aortitis: Takayasu's (pulseless disease) and temporal arteritis (giant cell arteritis) are the two most common arteritides to involve the aorta. Takayau's more commonly involves the aorta. Both disorders affect women most commonly- Takayasu's affecting women in their teens to thirties, and temporal arteritis affecting older women. Both aneurysm and stenosis are formation are common. Involvement of the aortic root is also common when there is aneurysmal dilatation and hence, regurge is also seen. The disorders typically produce associated thickening of the aortic wall.
b) Infectious Aortitis (also referred to as Mycotic aneurysm): The most common organisms are Staphylococcus (older males), Streptococcus, Pneumococcus, or Salmonella (younger or immune compromised patients). Etiologies include post-aortic or coronary artery bypass surgery. Prognosis is grim in the absence of antibiotic or surgical treatment. Mycotic aneurysms are usually saccular and contain eccentric thrombus [17]. CT and MR findings indicative of an infectious aortic aneurysm include wall thickening (with or without nodularity), periaortic inflammatory changes resulting in thickening of the periaortic tissues, perianeurysmal gas, and a saccular aneurysm (although, fusiform aneurysms can also occur).
Syphilis is a sexually transmitted disease caused b the
spirochete T palladium [23]. Tertiary syphilis
can involve the cardiovascular system, typically 10-20 years
after the initial infection [23,41]. Syphilitis
aortitis is felt to be an
inflammatory response to T palladium and causes focal
destruction of the media due to an endarteritis of the vasa vasorum
with loss of elastic and smooth muscle fibers and scarring
leading to aortic dilatation and aneurysms [17,23,41]. Syphilitic aortitis
is reported to occur in 70-80% of all cases of untreated
syphiltic infection and characteristically involves the
ascending aorta (60% of cases) followed by the aortic arch (30%
of cases) [23,41]. Aortic aneurysms are detected clinically in
only 5-10% of patients - most commonly (50%) involving the
asceding aorta, followed by the aortic arch (35%), and
descending aorta (15%) [41]. The majority of the are saccular,
but up to one-third can be fusiform [41]. Syphilitis
aneurysms are at high risk for rupture (up to 40% of cases)
[17]. On plain film, "pencil-line" fine calcifications within
the wall of the vessel are considered classic. [23].Other
complications include aortic insufficiency (seen in 20-30% of
patients with syphilitic aortitis) and coronary ostial stenosis
(seen in 20-26% of patients) [41].
4- Aortic stenosis:
Post-valvular dilatation of the ascending aorta is seen in association with aortic stenosis. There is relative preservation of the aortic root and sinotubular junction and the dilatation is usually limited to the mid ascending aorta where the post-stenotic flow effects are most pronounced.
5- Aortic insufficiency:
Insufficiency may also result in dilatation of the ascending aorta- likely related to the "water-hammer" effect. Dilatation in these cases is more likely to extend into the transverse arch, and there is less preservation of the aortic root and sinotubular junction.
|
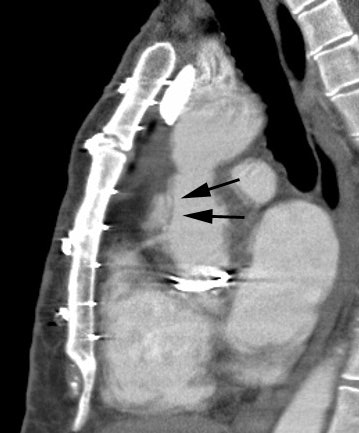
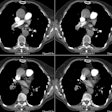
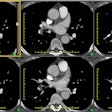
Hx graft repair of an ascending aortic aneurysm: A routine followup CT demonstrated a pseudoaneurysm arising from just above the right coronary artery graft site (black arrows). Surgical repair was performed. There was no evidence for infection. Click image to view cine file- note large amount of low attenuation between graft and native aorta. |
|
|
Abdominal aortic aneurysm:
Abdominal aortic aneurysms are defined as dilatation of the
abdominal aorta greater than 50% of the normal proximal segment
or a diameter greater than 3 cm [37]. The most accurate
measurements of the aneurysm diameter are obtained orthogonal to
a center line through the aorta [35]. An AAA is defined by its
location relative to the renal arteries [37]. An infrarenal AAA
arises at least 10 mm below the renal arteries, a juxta renal
AAA extends to the renal arteries, and a suprarenal AAA invovles
the renal arteries and extends superiorly [37]. The risk of
rupture increases with increasing diameter [37].
Abdomninal aortic aneurysms are more common than TAAs and occur in approximately 5% of screened males over 65 years of age and are often defined as dilatation ≥ 3 cm [45]. Concomitant abdominal aortic aneurysms occur in more than a quarter of patients with TAAs [43]. Abdominal aortic aneurysms expand at a rate of 2-4 mm per year when smaller than 4 cm, 2-5 mm per year when they are between 4-5 cm, and 3-7 mm per year when larger than 5 cm [9]. The risk for rupture of an abdominal aortic aneurysm is between 10-20% when it reaches 5-6 cm, and increases to 30-50% when it equals or exceeds 8 cm [19].
Series have shown that about 40% of aneurysm with diameters
larger than 5 cm will ruture over a 5 year period [38]. The
rupture risk has been reported as 1-3% per year at a diameter of
4-5 cm (other authors suggest less than or equal to 1% for a
diameter of 5.5 cm or less [43]), 6-11% per/yr between 5-7 cm
(other authors suggest 9% for diameter 5.5-5.9 cm and 10% for
diameter 6-6.9 cm [43]), and 20-33% per year when equal to or
greater than 7 cm [37,43]. Most abdominal aortic aneurysms grow
1-4 mm per year, and rupture risk versus oeprative risk is
blanaced at a 5.0-5.5 cm threshold for intervention [35].
Abdominal aortic aneurysms smaller than 5.5 cm are usually
followed with serial imaging at 6 month to 3 year intervals - a
12 month interval is recommended for aneurysms between 4-4.9 cm
and 3 year surveillance for those 3.0-3.9 cm [44].
Surgical thresholds for aneurysm repair varying depending on
the location of the aneurysm, but most surgeons will electively
repair typical fusiform abdominal aortic aneurysms that exceed
5.4 cm in greatest diameter or an aneurysm size that is 2.5
times the normal aortic diameter [16,35,37]. Surgical repair is
also indicated for aneurysms that enlarge more than 5-7 mm
within 6 months or 1 cm or more within one year [35]. Other
authors suggest guidelines generally recommend elective surgical
repair for AAA greater than 5-5.5 cm or expansion of more than
5-10 mm over a 6- to 12 month period [43]. Maximum cross
sectional aneurysm diameter, the thickness/extent/volume of
intralumenal thrombus, and a rapid increase in thrombus area are
independently associated with greater risk for more rapid
aneurysm growth [44].
Risk factors for rupture include active tobacco use,
uncontrolled hypertension, prior cardiac or renal transplant,
and female gender [43]. CT signs that are suggestive of
impending rupture include a hyperattenuating cresent sign, wall
irregularity, a new saccular outpouching/penetrating ulcer, the
draped aorta sign, and a periaortic hematoma [35]. The
hyperattenuating cresent sign refers to a perilumenal
curvilinear area of hyperattenuation (higher than intralumenal
blood at unenhanced CT) within the wall or thrombus of the aorta
[35]. Ruptured anueyrsms have been shown to contain less mural
thrombus and thrombus calcification compared with more stable
aneurysms [35]. Decreasing thrombus volume with progressive
enlargement of the flow lumen or new eccentric outpouching of
the lumen likely indicated lysis of thrombus and is another risk
factor for rupture [35]. The draped aorta sign is an indication
of contained rupture and refers to the posterior aortic wall
closely following or "draping" along the contour of the adjacent
vertebral body [35]. The aorta may also appear indistinct from
the vertebral body or psoas muscle, with loss of the periaortic
fat planes [35].
Endovascular stent grafting:
Endovascular stent grafting is an alternative to surgical
repair primary applied to the treatment abdominal aortic
aneurysms and for thoracic aortic aneurysms in patients who are
poor surgical candidates [15,16].
Endovascular repeair is best suited for infrarenal AAAs and a
1.5 cm landing zone of normal anatomy is required for infrarenal
fixation [37]. A short aneurysmal neck will result in radial
force exerted over a smaller area resulting in a greater risk of
inadequate seal, distal stent migration, and type 1 endoleak
[37]. Suprarenal fixation requires a bare metallic stent
component to extend above the fabric-covered stent graft [37].
The junction between the bare metallic stent and stent graft is
placed just below the renal arteries- this allows perfusion to
the superior mesenteric and renal arteries [37]. Tortuous AAA
with proximal neck angles of less than 120 degrees pose a
challange for proper delivery and deployment of the device [37].
Other factors of the proximal nexk that affect proper seating of
the device include excessive calcification or thrombus (more
than 2 mm thick or more than 50% circumferential involvement)
and diameter of the proximal neck (more than 28 mm) [37]. A
minimal iliac artery outer diameter of 7 mm is needed for device
delivery [37]. Other authors suggest hostil neck configurations
include a diameter of > 32 mm (which can result in proximal
seal or fixation failure), a length < 15 mm (which can result
in seal zone failure), angulation > 60 degrees (which can
result in incomplete circumferential wall apposition, and a
conical configuration (which can result in seal zone failure)
[45].
Immediately after endovascular stent placement, CTA imaging may
show aortic wall thickening and low density periaortic fluid [16].
Generally, aneurysms will decrease in size following successful endograft repair, however, a slight increase in size of the anuerysm sac may sometimes be seen on the first post-operative scan [16]. As long as no endoleak is identified, conservative management and careful followup can be performed [16]. The left subclavian artery may occasionally be occluded by the stent in order to achieve a minimum of 2 cm length of non-disease aorta for stent anchorage [16]. In these cases, retrograde flow from the left vertebral artery provides perfusion to the left arm [16]. Prior to occlusion of the vessel, cerebral arterial supply should be evaluated to ensure adequate posterior fossa circulation.
For repair of thoracic aortic aneurysms, patients with endovascular stent grafts have been suggested to have a lower prevalence of spinal cord ischemia, a lower prevalence of renal and respiratory insufficiency, and a shorter hospital stay [15]. Patient survival at two years is similar for open repair and stent graft patients [15]. The prevalence of endoleak for thoracic aortic aneurysms has been reported to be 6% at one year, and 9% at two years [15].
Complications of endovascular stent graft include: Endoleaks, stent collapse, stent
migration, pseudoaneurysm
formation, dissection, aortic perforation, kinking, thrombosis,
and coverage of branch vessels [15,16].
Undersizing of the endograft is associated with an increased
risk for endoleak or stent migration [24]. A certain degree of
oversizing has been shown to be beneficial by providing improved
fixation and sealing, however, excessive oversizing can
contribute to endograft infolding or dilatation with subsequent
device migration [24]. Generally, it is recommended that the
range of oversizing be 10-20% of the preoperative aortic
diameter at the aneurysm neck [24]. Some authors have suggested
the use of ECG-gated CT imaging to measure the neck of the
aortic aneurysm as the size of the aorta varies with systole
versus diastole [24].
1- Endoleak- Endoleak is the most common complication of stent graft repair. An endoleak is defined as blood flow external to the stent-graft and inside the aneurysm sac [13]. Long-term surveillance of patients following stent graft repair of AAA is required as endoleaks can be both and early and late complication of the procedure [14]. Leak can occur in up to 45% of patients (8% to 45% [7,29]; 15-52% of abdominal aortic aneurysms [12,14]; and up to 29% of thoracic aortic aneurysms [16]). Endoleaks are more consistently identified by multiphasic helical CT than conventional angiography [5,6]. This is because endoleaks have variable flow rates and are often detected at variable times following contrast administration [13]. Typically for the CT evaluation of endoleak, non-contrast, arterial phase, and late-phase images are obtained. Endoleaks seen only on delayed phase images may be more likely to close spontaneously [14].
MR imaging may be superior to CT for the detection of endoleak [11]- however, there are certain limitations to MR. Patients with stainless steel stent grafts should not undergo MR imaging because of the risk of migration or deformation of the graft by the strong magnetic field and extensive artifact associated with this type of stent [13]. Elgiloy stents can also obscure the vessel lumen [13]. Stents composed of nitinol are generally more suited to MR imaging [13].
There are 5 types of endoleaks [7]:
Type 1: The leak occurs at the graft insertion sites (ends of the graft) due to an inadequate seal between the stent graft and the aortic wall (i.e.- there is a separation between the stent graft and the native arterial wall [20]). The can be further classified as type Ia (proximal end of the graft) or type Ib (distal) [13]. These leaks result in elevated sac pressure and a continued risk for rupture (1% per year) [32,34]. Type I endoleaks are the most common to occur after endovascular repair of thoracic aortic aneurysms (they account for 40% of all endoleaks involving the thoracic aorta) [13,16]. Patients with severe angulation at the neck of the aneurysm are at an increased risk to develop proximal perigraft endoleaks [14,34]. These leaks usually appear as broad-based collections directly adjacent to the prosthesis [12]. Large circumferential perigraft collections are indicative of dislocation of the stent-graft or insufficient length a tube endoprothesis [12]. Type I endoleaks are repaired immediately following diagnosis by securing the graft attachment sites with angioplasty balloons, extending the stent coverage with additional stents, or stent graft extensions [13,16,17].
Type 2: The leak occurs when there is retrograde inflow into the aortic sac via a patent branch vessel and it is the most common type of endoleak (observed in 17-23% of patients, up to 30%) [10,12,14,25,31,36,37]. Type II endoleaks can also impart systemic or near systemic pressure within the excluded zone of the aneurysm due to the retrograde flow via the branch vessel and result in continued aneurysm sac expansion [32,35,40]. In type IIA endoleaks (simple endoleak), there is a single feeding branch vessel, while two or more feeding branch vessels indicate a Type IIB endoleak (complex endoleak) [16,31,36]. An endoleak detected within 90 days after EVAR is defined as an early endoleak, and one detected after 90 days is defined as a late endoleak [36]. A transient endoleak will resolve spontaneously within 6 months, and the endoleak is considered persistent if it lasts more than 6 months [40].
For abdominal aortic aneurysms, typical sources include the inferior mesenteric artery and the lumbar arteries [13,14,36], other potential sources include the median sacral artery or even accessory renal arteries [36]. For thoracic aneurysms, feeding vessels include bronchial and intercostal arteries, a patent ductus arteriosus, and the subclavian arteries [16]. For abdominal aortic aneuysms- ventral collections without direct connection to the endoprosthesis are supplied by the inferior mesenteric artery (persistent inflow from the IMA is responsible for 45-85% of AAA endoleaks [31]), dorsolateral collections are supplied by the lumbar or median sacral artery [13]. Retrograde endoleaks are unavoidable with current endovascular techniques [8]. The risk for type 2 endoleak is increased with increasing patent side branch vessels [7,31]. Other risk factors for a persistent endoleak include early appearance of the endoleak on the final operative angiogram (within 6 seconds) and high attenuation of the leak on the first post operative CT scan [40]. For abdominal endografts, the diameter of the IMA does not seem to necessarily be associated with an increased risk for endoleak [31].
The clinical importance and management of Type 2 endoleaks is not clear. Type II endoleaks (particularly small leaks- less than 15 mm) that have a stable or decreasing aneurysm sac size can be treated conservatively and followed with serial CT evaluation as they have a high rate of spontaneous resolution and a low risk of rupture (more than half of type II endoleaks will spontaneously resolve within the first 6 months) [10,12,13,14]. However, this type of leak (even small leaks) is often associated with failure of the aneurysm to decrease in size and it may actually increase due to persistent pressurization of the aneurysm sac [31]. Treatment should be performed if the feeding vessel is large, if there is significant contrast enhancement in the excluded aortic lumen, if the sac diameter grows by more than 1 cm over 1 year, or the excluded aortic lumen demonstrates progressive enlargement [16,29,45]. An endoleak cavity diameter measuring more than 1.42 cm has been suggestive of a greater likelihood for eventual surgical intervention [25]. Other factors associated with a higher risk for aneurysm sac enlargement include a higher number of feeding vessels (complex endoleak) and if the largest feeding vessel was 4 mm or more in diameter (a 91% risk for aneurysm sac enlargement) [36].
Treatment involves embolization of the culprit vessel near its communication with the aneurysm sac to block the retrograde flow of blood [13]. Unfortunately, coil embolization for IMA related endoleaks can fail in up to 80% of cases [31]. Preoperative embolization of the IMA has been introduced to reduce the risk of type II endoleak, but this is time consuming and expensive and places the patient at additional risk [31].
Type 3 leaks occur due to a structural failure of the stent graft including component disconnection, fabric tears/fracture of the metallic skeleton, and disintegration of graft material. Junctional dehiscence results from a defect between two adjacent or overlapping stents and often occurs early following technically complex stent procedures [16]. Type III endoleaks are currently fairly unusual, but may become more common during long-term followup [13]. Type 3 endoleaks are treated immediately with a stent graft extension because they represent a direct communication systemic arterial blood with the aneurysm sac [13,17].
Type 4 leaks occur due to transgraft flow due to graft wall porosity. This is an exceedingly rare endoleak and are self limited [12,13]. It is identified at the time of implantation as a blush on the post implant angiogram when patients are fully anticoagulated [13]. These endoleaks require no specific intervention other than normalization of the coagulation profile [13].
Type 5 or "endotension"- no leak is visible radiographically, but the aneurysm continues to grow [5]. This may be because the blood flow is undetectable by standard imaging, because of pressure transmission through the fabric, or serous ultrafiltrate across the endograft fabric [45]. Scanning the graft in both the arterial and delayed phases is important to make sure that a subtle leak is not missed. Type 5 endoleaks carry a long-term risk for sac rupture [35]. Repair is indicated if the sac diameter increases by more than 1 cm [45].
About 18% of leaks will only be seen on arterial phase images, while about 3% will only be seen on delayed exams. Note that gas may be seen in the aneurysm sac immediately following stent deployment and should not be considered pathologic when scans are performed at that time. For thoracic aortic endografts, a "bird-beak" configuration (defined as an incomplete apposition of the proximal endograft with a wedge-shaped gap between the device and the aortic wall) is associated with a markedly increased risk for Type Ia or IIa endoleak [21].
2- Graft thrombosis: Partial, peripheral, or semicirular thrombosis is seen in 3-19% of stents [10]. Graft occlusion is rare [6].
3- Graft kinking: Kinking occurs when large aneurysms shrink/shorten after stent grafting [13]. The kinking may not be detected on transaxial images, but can be demonstrated on MIP or MPR images. Kinking is in turn associated with graft migration, thrombosis, and endoleak [13].
4- Graft migration: Occurs due to poor attachment of the stent to the aortic wall [16]. Migration of 5 mm or more is considered substantial and stent graft position with respect to a constant anatomic landmark should be recorded during followup imaging [13].
5- Shower or peripheral embolism: Shower embolism occurs in 4-17% of cases and is generally fatal [6]. Peripheral embolism can lead to organ or limb ischemia [10].
6- Colonic necrosis (abdominal aortic stent grafting)
7- Aortic dissection (2%) [10].
8- Vascular perforation
9- Pseudoaneurysm associated with graft infection: In the abdominal aorta, more than 5 mm of perigraft soft tissue between the aneurysm wall and the graft is abnormal and may suggest infection [22].
10- Stent collapse- predisposing factors are poor stent
attachment and oversizing of the
stent [16]. On CT there is narrowing of the endolumenal stent diameter and
displacement of the stent from the vessel wall [16]. This
complication requires urgent surgical intervention when there is
significant narrowing of the aortic lumen [16].
11- Suture breaks and metal-ring fractures: AneuRx stent grafts
were the most widely deployed stent graft up until 2012 when it
was replaced by a newer design [34]. The graft was constructed
from self-expanding nickel-titanium (nitinol) and woven
polyester graft material [34]. The diamond shaped nitinol
segments are laser cut from a single piece of nitinol tubing
[34]. As a result the grafts are predisposed to two types of
mechanical failure- fracture of the metallic stent rings and
breakage of the polyester sutures that connect adjacent rings
[34]. Suture breaks and metal-ring fractures are associated with
type I (due to graft migration) and III (due to component
separation) endoleaks [34]. Major suture breaks and metal-ring
fractures occur most frequently at the junction between the main
body and the limbs of the bifurcated graft [34].
12- Post endograft repair aortic rupture: The rate of post
endograft repair aortic rupture ranges from 0.4 % to 1.1% with
the time to rupture ranging from 3 days to 85 months [35]. Risk
factors for post endograft instability include graft migration
more than 5mm, graft kinking or fracture, and persistent
endoleaks [35].
13- Renal infarct: Renal infarction can be seen in up to 26% of
patients following stent graft repair [42]. Often (up to 39% of
cases) this is the result of intentional exlcusion of a small
accessory renal artery [42]. However, up to 61% can be related
to embolic phenomena [42]. Other causes include a flow limiting
renal artery dissection [42].
REFERENCES:
(2) Magn Reson Imaging Clin N Am 1996; 4(2): 217-235
(3) Society of Thoracic Radiology Annual Meeting Course Syllabus 1997; 21-25
(4) Radiology 1997; 203: 727-732
(5) Radiology 2000; Armerding MD, et al. Aortic aneurysmal disease: Assessment of stent-graft treatment- CT versus conventional angiography. 215: 138-146
(6) Radiographics 2000; Mita T, et al. Complications of endovascular repair for thoracic and abdominal aortic aneurysm: An imaging spectrum. 20: 1263-1278
(7) Radiology 2001; Fan CM, et al. Endovascular stent-graft in abdominal aortic aneurysms: The relationship between patent vessels that arise from the aneurysmal sac and early endoleak. 218: 176-182
(8) Radiology 2001; Gorich J, et al. Endoleaks after endovascular repair of aortic aneurysm: are they predictable?- Initial results. 218: 477-480
(9) AJR 2003; Macura KJ, et al. Pathogenesis in acute aortic syndromes: aortic aneurysm leak and rupture and traumatic aortic transection. 181: 303-307
(10) Radiology 2005; Tolia AJ, et al. Type II endoleaks after endovascular repair of abdominal aortic aneurysms: natural history. 235: 683-686
(11) AJR 2005; Pitton MB, et al. MRI versus helical C or endoleak detection after endovascular aneurysm repair. 185: 1275-1281
(12) Radiology 2006; Chernyak V, et al. Type II endoleak after endoaortic graft implantation: diagnosis with helical CT arteriography. 240: 885-893
(13) Radiology 2007; Stavropoulos SW, Charagundia SR. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. 243: 641-655
(14) AJR 2008; Hong C, et al. CLinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. 191: 808-813
(15) Radiographics 2008; Bean MJ, et al. Thoracic aortic stent-grafts: utility of multidetector CT for pre- and postprocedure evaluation. 28: 1835-1851
(16) AJR 2009; Hoang JK, et al. MDCT angiography of thoracic aorta endovascular stent-grafts: pearls and pitfalls. 192: 515-524
(17) Radiographics 2009; Agarwal PP, et al. Multidetector CT of thoracic aortic aneurysms. 29: 537-552
(18) Radiographics 2009; Chao CP, et al. Natural hsitory and CT appearance of aortic intramural hematoma. 29: 791-804
(19) AJR 2009; Litmanovich D, et al. CT and MRI in diseases of the aorta. 193: 928-940
(20) Radiographics 2010; Kimura-Hayama ET, et al. Uncommon congenital and acquired aortic diseases: role of multidetector CT angiography. 30: 79-98
(21) Radiology 2010; Ueda T, et al. Incomplete endograft apposition to the aortic arch: bird beak configuration increases risk of endoleak formation after thoracic endovascular aortic repair. 255: 645-652
(22) Radiographics 2010; Morgan TA, et al. Acute traumatic aortic injuries: posttherapy multidetector CT findings. 30: 851-867
(23) Radiographics 2011; Restrepo CS, et al. Aortitis: iimng
spectrum of the infectious and inflammatory conditions of the
aorta. 31: 433-451
(24) Radiology 2011; Iezzi R, et al. Proximal aneurysmal neck:
dynamic ECG-gated CT angiography - conformational pulsatile
changes with possible consequences for endograft sizing. 260:
591-598
(25) AJR 2011; Keedy AW, et al. Evaluation of potential outcome
predictors in type II endoleak: a retrospective study with CT
angiography feature analysis. 197: 234-240
(26) AJR 2012; Chu LC, et al. Vascular complications of
Ehlers-Danlos syndrome: CT findings. 198: 482-487
(27) Radiographics 2012; Bennett CJ, et al. CT and MR imaging
of the aortic valve: radiologic-pathologic correlation. 32:
1399-1420
(28) Radiographics 2013; Prescott-Focht JA, et al. Ascending
thoracic aorta: postoperative imaging evaluation. 33: 73-85
(29) Radiology 2013; Lehmkuhl L, et al. Dynamic CT angiography
after abdominal aortic endovascular aneurysm repair: influence
of enhancement patterns and optimal bolus timing on endoleak
detection. 268: 890-899
(30) AJR 2013; Chu LC, et al. MDCT evaluation of aortic root
surgical complications. 201: 736-744
(31) Radiology 2014; Guntner O, et al. Inferior mesenteric
arterial type II endoleaks after endovascular repair of
abdominal aortic aneurysm: are they predictable? 270: 910-919
(32) AJR 2014; Maddu KK, et al. Nontraumatic acute aortic
emergencies: Part 2, pre- and postsurgical complications related
to aortic aneurysm in the emergency clinical setting. 202:
666-674
(33) AJR 2014; Chu LC, et al. CT angiographic evaluation of
genetic vascular disease: role in detection, staging, and
management of complex vascular pathologic conditions. 202:
1120-1129
(34) Radiology 2014; Ueda T, et al. Detection of broken sutures
ad metal-ring fractures in AneuRx stent-grafts by using
three-dimentional CT angiography after endovascular abdominal
aortic aneurysm repair: association with late endoleak
development and device migration. 272: 275-283
(35) Radiographics 2015; Wadgaonkar AD, et al. Abdominal aortic
aneuryms revisited: MDCT with multiplanar reconstructions for
identifying indicators of instability in the pre and post
operative period. 35: 254-268
(36) Radiology 2015; Muller-Wille R, et al. CT features of
early type II endoleaks after endovascular repair of abdominal
aortic aneurysms help predict aneurysm sac enlargement. 274:
906-916
(37) Radiographics 2015; Bryce Y, et al. Endovascular repair of
abdominal aortic aneurysms: vascular anatomy, device selection,
procedure, and procedure-specific complications. 35: 593-615
(38) J Nucl Med 2015; Rudd JHF, et al. Preciting aortic
aneurysm expansion by PET. 56: 971-973
(39) Radiographics 2016; Hanneman K, et al. Pre- and
postoperative imaging of the aortic root. 36: 19-37
(40) AJR 2016; Mursalin R, et al. Imaging-based predictors of
persistent Type II endoleak after endovascular abdominal aortic
aneurysm repair. 206: 1335-1340
(41) Radiographics 2017; Lian K, et al. Syphilitic aortitis
with coronary ostial involvement. 37: 407-412
(42) AJR 2017; Burke LMB, et al. Incidence and clinical
significance of renal infarct after fenetrated endovascular
aortic aneurysm repair. 208: 885-890
(43) J Nucl Cardiol 2017; Malm BJ, Sadeghi MM. Multi-modality
molecular imaging of aortic aneurysms. 24: 1239-1245
(44) Radiology 2020; Zhu C, et al. Intralumenal thrombus
predicts rapid growth of abdominal aortic aneurysms. 294:
707-713
(45) AJR 2020; Smith T, Quencer KB. Best practice guidelines:
imaging surveillance after endovascular aneurysm repair. 214:
1165-1174