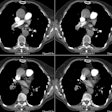
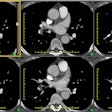
Chronic Pulmonary Embolic Disease:
View cases of chronic PE
Clinical:
In most patients with acute pulmonary embolism there is total resolution or resolution with minimal residua and restoration of normal pulmonary hemodynamics within 30 days after treatment [22]. For reasons unclear, in about 4-5% of patients with pulmonary emboli, the thrombi fail to lyse completely and become organized which results in varying degrees of vascular occlusion [22]. If more than 60% of the cross-section of the pulmonary arterial bed is occluded from those unresolved thrombi, progressive secondary pulmonary hypertension develops. Pulmonary hypertension (pulmonary artery pressure greater than 25 mm Hg at rest, or 30 mm Hg at exercise) develops in 0.01 to 9.1% of patients with chronic thromboemboli [15,27]. Other authors state that pulmonary hypertension as a result of chronic PE (CTEPH) can develop in up to 3.8% of patients after acute pulmonary embolism at 2 year followup [21,26]. The presence of a completely occlusive clot in a central or lobar pulmonary artery on initial imaging, proximal pulmonary embolism, and older age have all been associated with an increased risk for developing CTEPH [27]. As many as 75% of patients with newly diagnoses CTEPH have a history of acute PE and 56% of patients have a history of DVT [26]. However, other authors state that up to 63% of patients with CTEPH have no previous history of documented PE [21].Antiphospholipid antibody (APA) syndrome can be a cause of chronic pulmonary emboli. Up to 10-24% of patients with chronic PE's have elevated APA titers [9]. Lupus anticoagulant (a prothrombotic factor) is detected in about 10% of patients with chronic PE and 20% carry anticardiolipin antibodies, lupus anticoagulant, or both [22].
The most common presenting symptom is indolent, but progressive
shortness of breath with normal PFT's. Patients with chronic PE
associated pulmonary hypertension become symptomatic only when at
least 60% of the pulmonary arterial bed is obstructed [24].
Pulmonary thromboendarterectomy (PEA) is the definitive treatment
for chronic thromboembolic pulmonary hypertension with excellent
short and long term outcomes [27] (patients with pulmonary
arterial hypertension secondary to chronic PE may have their
symptoms ameliorated with pulmonary thromboendarterectomy). A
favorable prognosis following surgery is suggested in those
patients in whom CT demonstrates predominantly central vessel
occlusive disease (main, lobar, and proximal segmental arteries)
and limited small vessel involvement [13,22], but some patients
with predominantly peripheral (segmental) disease can also
experience improvement. The presence of dilated bronchial arteries
is also positively correlated with a lower mortality rate after
pulmonary thromboendarterectomy [22]. Criteria for inoperability
include distal pulmonary artery obstructions, imbalance between
increased pulmonary vascular resistance and the number of
accessible occlusions suggesting microvascular disease, a
pulmonary resistance greater than 1500 dyn-sec-cm-5,and
comorbidity [34]. Poor subpleural perfusion in the capillary phase
of digital subtraction angiography also predicts worse outcomes
following PEA in operable candidates [34]. Poor subpleural
perfusion is defined as less than or equal to 1.5 cm
(approximately one rib width) from the lateral pleura in the
capillary phase od digital subtraction angiography on the PA views
[34].
Thromboenarterectomy involves removal of both the thrombus and
the intima of the involved vessel. Patient response is usually
dramatic with a decrease in pulmonary vascular resistance and
decreased dyspnea. After PEA surgery, a reduction in the PA
pressure to less than or equal to 25 mm Hg at rest is achieved in
about 50% of patients; another 30% achieve nearly normal pulmonary
hemodynamics with a mean PA pressure between 25-30 mm Hg [34].
Normalization or near-normalization of pulmonary hemodynamics
after PEA (or BPA) is usually accompanied by substantial
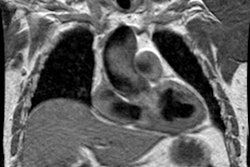
symptomatic improvement [34]. Following successful PEA, cine MR
can demonstrate normalization of the right and left ventricular
end-systolic and end-diastolic volumes, re-establishment of
interventricular synchrony, a return of the "leftward" ventricular
septal bowing, and a decrease in the RV mass [34]. Reverse cardiac
remodeling occurs within the first 4 weeks after PEA with fewer
changes at 3 or 6 months after surgery [34].
The mortality rate from the procedure, however, is high (4-8%) [21]. Complications of the procedure include inadequate endarterectomy, post thromboendarterectomy reperfusion edema [11], and phrenic nerve paralysis. Without treatment the prognosis is very poor with only a 30% 5 year survival when the mean pulmonary artery pressure is greater than 30 mm Hg [21].
Reperfusion edema occurs in almost all patients following
thromboendarterectomy for chronic pulmonary embolism. It is
characterized by patchy bilateral perihilar alveolar opacities
with maximum opacification generally seen by the second
post-operative day. The opacities generally resolve within 2
weeks. There is no correlation with preoperative pulmonary
arterial pressures and no correlation between the pre-operative
location of thrombus and post-operative opacities [11].
Balloon angioplasty (BPA) is an alternative therapy for patients
with inoperative CTEPH [34]. The success rate is higher, and the
complication rate lower, in ring-like stenosis and web lesions
[34]. Totally occlusive lesions have the lower success rate and
tortuous lesions have the highest complication rate [34]. Poor
subpleural perfusion in the capillary phase of pulmonary
angiography is another predictor of BPA failure [34].
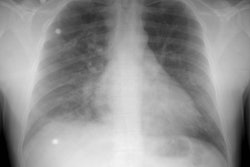
X-ray:
Computed Tomography:
CT exam:An unenhanced chest CT scan may be acquired initially using narrow collimation (1 mm) and a 10 mm gap to allow better evaluation of the lung parenchyma. A contrast exam is then performed from the level of the aortic arch to the base of the left atrium. The scan is performed from the diaphragm towards the arch (caudocranial direction) during an infusion of 140 ml of 30% contrast material at 2-3 ml/sec. The delay between the start of contrast injection and image acquisition may need to be delayed due to underlying pulmonary hypertension and decreased right ventricular function. A smart prep test injection can be used to time the exam for maximal pulmonary artery opacification. Scanning caudo to cranial and using a lower injection rate reduces streak artifacts from concentrated contrast material in the superior vena cava which may obscure the adjacent right main pulmonary artery. Scanning in this manner may also help to decrease vessel degradation due to breathing artifact [6].
Exam interpretation:
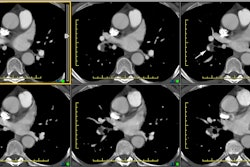
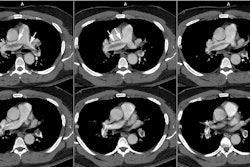
The most important finding on CT is visualization of organized embolic material which appears as an eccentric, broad-based crescentic (forming obtuse angles with the vessel walls), mural-adherent filling defect in the pulmonary arteries [16,19]. The thrombus may contain calcifications in up to 10% of cases. In general, the HU attenuation of chronic PE is significantly higher than that of acute PE (about 87 HU versus 33 HU, respectively) [18]. Other findings on CT include complete vessel occlusion (typically with a convex margin with respect to the contrast material producing a "pouch"-like defect [19]), variation/disparity in size of the segmental vessels (a vessel size ratio of greater than one based on comparison to the diameter of the corresponding contralateral segmental vessel at a similar level), vessel reduction of the overall arterial diameter of greater than 50% secondary to recanalization, and a band, web, or flap within a contrast filled artery (these appear as thin-lines surrounded by contrast within the vessel - most commonly lobar or segmental arteries [16,19,22]. An intravascular band is defined as a delicate ribbon-like structure (1-3 mm in width) oriented to the direction of flow and anchored to the vessel wall at two points (typically 0.3 to 2 cm in length) [19]. A web describes a more complex band with branches that forms a network of varying complexity [19].
Enlargement of the main pulmonary arteries (secondary pulmonary arterial hypertension) is suggested when the ratio of the diameter of the main pulmonary artery to the diameter of the ascending aorta is greater than 1:1 [22]. Pulmonary artery enlargement associated with chronic PE is often asymmetric (in contrast to the symmetric enlargement in non-thromboembolic PAH) [10]. Pulmonary artery wall calcification, right ventricular hypertrophy (RV myocardial thickness greater than 4 mm), and right ventricular/atrial enlargement (due to right heart failure) may be identified [22].
Bronchial artery enlargement (development of collateral vessels) may also be seen [10,16]. In patients that have developed PAH there is an enlargement of the bronchial circulation as a result of systemic-to-pulmonary anastomoses which help to maintain pulmonary blood flow [17]. The normal bronchial arterial flow is about 1-2% of the cardiac output, but this can increased to almost 30% in patients with chronic PE [22]. The bronchial arteries usually arise from the descending aorta at the level of the carina [22]. Abnormal dilatation (diameter more than 2 mm) and arterial tortuosity of the bronchial arteries are indicative of bronchial artery hypervascularization [22]. Abnormally enlarged bronchial and nonbronchial systemic arteries are found more frequently in patients with chronic thromboembolic pulmonary hypertension (73%), than in patients with idiopathic pulmonary hypertension (14%) [22]. Other authors indicate that dilated bronchial arteries (diameter ≥ 1.5 mm) are seen in 47-77% of cases and dilated non-bronchial collaterals (inferior phrenic, intercostal, and internal mammary arteries) are visible in up to 45% of cases [23].
Mosaic lung perfusion is seen in 77-100% of cases of recurrent thromboembolic disease- particularly patients with PAH [23]. In chronic pulmonary embolism, vascular occlusion of small arteries supplying secondary pulmonary lobules produces inhomogeneous attenuation (regional hypoperfusion and decreased lung attenuation) of the lung parenchyma or a "mosaic" pattern of attenuation or "mosaic oligemia" [1,2]. Vessels within the low attenuation regions have a smaller cross-sectional diameter compared to vessels within the regions of higher attenuation. These parenchymal changes are best appreciated on non-contrast thin collimation images. Mosaic oligemia may need to be distinguished from other causes of localized decreased lung attenuation such as regional air trapping- this can be accomplished with the use of expiratory images. Wedge shaped areas of peripheral infarction/scarring may also be identified in 10-15% of cases (other authors indicate that peripheral opacities caused by previous infarction can be seen in 72-87% of cases [23]).
Overall, helical CT is superior to conventional angiography in the detection of mural thrombi associated with chronic pulmonary embolism- especially with the use of reconstruction images along the coarse of the affected vessel. The sensitivity of conventional angiography in depicting chronic thromboembolic disease (particularly central disease) is likely due to concentric incorporation of the thromboembolic material into the vessel wall. This epithelialized thrombus has a smooth surface which may not be identified at angiography. [5,12] The sensitivity for CT in detecting chronic PE has been reported to be 70% for segmental and 64% for subsegmental branches [21]. In another study, CTPA imaging had a sensitivity of only 51% for the detection of CTEPH [21].
Mutliplanar images can also aid in determining operability for patients with chronic embolic disease [20]. A suitable endarterectomy plane is defined as intimal thickening of the pulmonary artery starting at the pulmonary artery trunks no further than the origin of the lobar arteries [20]. Patients in whom pulmonary artery thickening starts distal to the lobar origin are not candidates for endarterectomy [20].
Cylindrical bronchiectasis (bronchial dilatation) involving the segmental and sub-segmental bronchi within regions of chronic pulmonary embolism may also be identified [3].
A pulmonary artery sarcoma may potentially be misinterpreted as a thrombus, but the tumor will enhance following the administration of contrast and it typically distends the affected vessel. Takayasu's arteritis usually affects both the aorta and pulmonary arteries- in the rare event of isolated pulmonary involvement, the wall thickening is usually high attenuation and contrast enhancing.
Ventilation-Perfusion Scintigraphy:
Patients with chronic PE generally have abnormal V/Q scans read as high or intermediate probability [14,21]. V/Q imaging has a reported sensitivity of 94-97%, a specificity of 86-95%, and an accuracy of 93-95% in the evaluation of chronic pulmonary thromboembolic disease (depending on whether only high or high and intermediate probability exams are considered positive) [4,21]. V/Q scanning has been shown to be more sensitive than CTPA imaging for the detection of chronic thromboembolic disease [21]. Scintigraphic findings include multiple segmental perfusion defects. Unilateral hypoperfusion or predominantly unilateral hypoperfusion can also be identified [12]. A normal V/Q scan practically rules out the presence of chronic thrombotic pulmonary hypertension (NPV is about 98%) [21,22,25,28].REFERENCES:
(3) Radiology 1997; 203: 355-360
(5) Radiology 1997: 204: 695-702
(6) J Thorac Image 1997; 12: 118-127
(7) J Thorac Image 1997; Reply to opinions.12: 100-102 (No abstract available)
(8) J Thorac Image 1997; 12: 103-117
(9) AJR 1998; Provenzale JM, et al. Systemic thrombosis in patients with antiphospholipid antibodies: Lesion distribution and imaging findings. 170: 285-290
(10) AJR 1998; King MA, et al. Chronic thromboembolic pulmonary hypertension: CT findings. 955-960 (No abstract available)
(11) J Thorac Imaging 1998; Miller WT, et al. Reperfusion edema after thromboendartectomy: Radiologic patterns of disease. 13: 178-183
(12) Radiology 1999; Bergin CJ, et al. Identifying the cause of unilateral hypoperfusion in patients suspected to have chronic pulmonary thromboembolism: Diagnostic accuracy of helical CT and conventional angiography. 213: 743-749
(13) AJR 2000; Bergin CJ, et al. Predictors of patient response to pulmonary thromboendarterectomy. 174: 509-515
(14) Radiographics 2000; Frazier AA, et al. From the archives of the AFIP. Pulmonary vasculature: Hypertension and infarction. 20: 491-524
(15) J Nucl Cardiolo 2003; Boyce PD, et al. Pulmonary hypertension: work in progress. 10: 413-423
(16) Radiographics 2004; Wittram C, et al. CT angiography of pulmonary embolism: diagnostic criteroa and causes of misdiagnosis. 24: 1219-1238
(17) Radiology 2005; Remy-Jardin M, et al. Systemic collateral supply in patients with chronic thromboembolic and primary pulmonary hypertension: assessment with multi-detector row helical CT angiography. 235: 274-281
(18) Radiology 2005; Wittram C, et al. Attenuation of acute and chronic pulmonary emboli. 235: 1050-1054
(19) AJR 2006; Wittram C, et al. Acute and chronic pulmonary emboli: angiography-CT correlation. 186: S421-429
(20) AJR 2007; Paul JF, et al. Findings on submillimeter MDCT are predictive of operability in chronic thromboembolic pulmonary hypertension. 188: 1059-1062
(21) J Nucl Med 2007; Tunariu N, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic disease as a treatable cause of pulmonary hypertension. 48: 680-684
(22) Radiographics 2009; Castaner E, et al. CT diagnosis of chronic pulmonary thromboembolism. 29: 31-53
(23) Radiographics 2010; Grosse C, Grosse A. CT findings in
diseases associated with pulmonary hypertension: a current review.
30: 1753-1777
(24) Radiographics 2012; Pena E, et al. Pulmonary hypertension:
how the radiologist can help. 32: 9-32
(25) J Nucl Cardiol 2015; Ohira H, et al. The role of nuclear
imaging in pulmonary hypertension. 22: 141-157
(26) AJR 2017; Grosse A, et al. Distinguishing chronic
thromboembolic pulmonary hypertension from other causes of
pulmonary hypertension using CT. 209: 1228-1238
(27) AJR 2020; Lorenz G, et al. CT-based biomarkers for
prediction of chronic thromboembolic pulmonary hypertension after
an acute pulmonary embolic event. 215: 800-806
(28) Radiology 2021; Remy-Jardin M, et al. Imaging of pulmonary hypertension in adults: a position paper from the Fleischner society. 298: 531-549