Nano-X Imaging (Nanox) has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Nanox.ARC X, the company's new multisource digital tomosynthesis system.
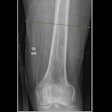
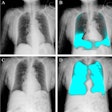
The clearance covers the production of tomographic images for general use, including the human musculoskeletal system and pulmonary, intra-abdominal, and paranasal sinus indications, as an adjunct to conventional radiography on adult patients.
The Nanox.ARC X maintains the Nanox.ARC’s proprietary digital Nanox.Source and advanced tomosynthesis technology with a cold cathode. This allows the system to create a more comprehensive, sliced 3D view of the body, improve visualization with multiple layers of images, and reduce the super-imposition of structures, the company highlighted.
The Nanox.ARC X introduces a fully integrated, single-unit system and features "plug and play" one-day installation capability, Nanox added. The system is designed to be installed in any standard x-ray shielded room with minimal infrastructure requirements. The system operates on standard power (110v/230v 16A) and features a cables-free design.
Nanox said it will offer the system later in 2025 alongside the current Nanox.ARC.