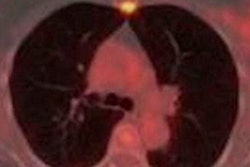
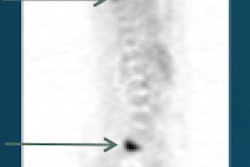
With the ability to accurately detect extraskeletal disease, PET/CT and whole-body MRI can change treatment plans for high-risk breast and prostate cancer patients, according to a study published in the December issue of the Journal of Nuclear Medicine.
Stanford University researchers also found the combined administration of F-18 sodium fluoride (NaF) and F-18 fluorodeoxyglucose (FDG) in a single PET/CT scan achieves significantly greater sensitivity and accuracy than alternative methods for the detection of skeletal lesions.
The prospective study included 15 women with breast cancer and 15 men with prostate cancer who were referred for standard-of-care bone scintigraphy. NaF/FDG PET/CT and whole-body MRI scans were performed after bone scintigraphy (JNM, December 2015, Vol. 56:12, pp. 1862-1868).
Researchers, led by Dr. Ryogo Minamimoto, PhD, then compared results of the combined use of NaF/FDG-PET/CT in patients with breast or prostate cancers with those obtained using technetium-99m (Tc-99m) methylene diphosphonate (MDP) bone scintigraphy and whole-body MRI.
Preliminary results found that NaF/FDG PET/CT is significantly more accurate in detecting skeletal lesions than whole-body MRI and Tc-99m MDP scintigraphy. NaF/FDG PET/CT and whole-body MRI also discovered extraskeletal disease that may change the way treatment is managed for these patients.
Minamimoto and colleagues also noted the combined administration of NaF/FDG in a single PET/CT scan provided appropriate staging of high-risk patients with prostate cancer greater than stage II or prostate-specific antigen (PSA) greater than 10, as well as breast cancer above stage III.
The authors added that bone scintigraphy likely will continue as the first option for skeletal metastases detection due to its low cost and high performance, while the other two modalities could target patients with negative or equivocal bone scans and high clinical suspicion for metastases.
Financial support for the study was provided by GE Healthcare.