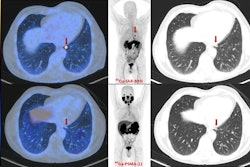
Radiopharmaceutical firm Clarity Pharmaceuticals said that its copper-64 SAR-Bombesin (SAR-BBN) agent has been used in the first patient of its Copper-64 Bombesin in Breast Cancer Trial (C-BOBCAT).
C-BOBCAT is the first in-human clinical trial investigating the use of the radiopharmaceutical in patients with hormone-receptor-positive/HER2-negative metastatic breast
cancer, according to the company. The trial is being led by Dr. Louise Emmett of St. Vincent's Hospital in Sydney, Australia.
"We hope that SAR-BBN will allow for [PET] imaging and localization of metastatic breast cancer lesions that express [gastrin-releasing peptide receptor (GRPr)], and we look forward to utilizing that data to progress SAR-BBN into other diagnostic and therapeutic trials in a range of cancers that express GRPr with our ultimate goal of better treating children and adults with cancer," said Clarity CEO Alan Taylor, PhD, in a statement.