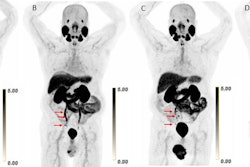
The Society for Nuclear Medicine and Molecular Imaging (SNMMI) is now considering all PSMA-PET radiotracers for detecting prostate cancer -- whether approved or not -- as equivalent, and it will refer to them as a class ("PSMA PET"), according to a recent update of the society's Appropriate Use Criteria (AUC).
"Although there may be small differences between each radiopharmaceutical, there is no evidence to date that one specific radiopharmaceutical has improved diagnostic characteristics compared with another," the SNMMI's AUC for Prostate-Specific Membrane Antigen PET Imaging states.
The increasing use of radiopharmaceuticals that target prostate-specific membrane antigen (PSMA) is based on growing scientific evidence that supports their favorable imaging, the guidelines state.
Currently, there are two radiotracers approved by the U.S. Food and Drug Administration (FDA) that target PSMA expressed in prostate cancer: F-18 DCFPyL (Pylarify, Lantheus Medical Imaging) and gallium-68 (Ga-68) PSMA-11.
Additional agents are being evaluated in phase III trials in the U.S., including the following:
- F-18 PSMA-1007
- F-18 rhPSMA-7.3
- F-18 CTT1057
- Ga-68 PSMA-R2
- Copper-64 (Cu-64) SAR-bisPSMA
The update comes one the heels of the FDA's approval of lutetium-177 (Lu-177) prostate-specific membrane antigen radioligand therapy (Pluvicto, Novartis) for the treatment of patients with metastatic prostate cancer.
In addition to the drug approval, the FDA approved Novartis' kit (Locametz) for the preparation of Ga-68 PSMA-11, which was used to identify metastatic prostate cancer tumors in patients during clinical trials.
The National Comprehensive Cancer Network added Ga-68 PSMA-11 and Pylarify to recommendations on the use of radiotracers in prostate cancer testing last year.