Seventeen years was all it took for flurpiridaz F-18 to reach the market – the first patient was injected with the PET imaging agent in November 2007, by Jamshid Maddahi, MD, and colleagues, of the University of California, Los Angeles.
Today, the radiotracer is poised to help expand the use of cardiac PET imaging on a national scale and ultimately improve care for people with coronary artery disease (CAD). Maddahi, who led clinical trials for F-18 flurpiridaz for its recent approval, discussed the impact the tracer may have on heart care in a recent interview.
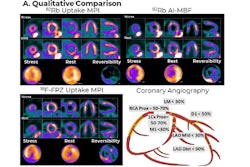
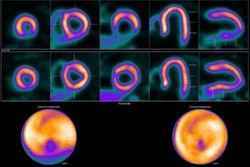
Maddahi noted that F-18 flurpiridaz proved effective in obese patients and women, which is significant given these groups are at higher risk for CAD. The tracer binds to key enzymes in the heart related to its ability to contract and relax, with abnormal uptake of the radiotracer indicating potential disease due to blocked arteries.
He added that aside from its proven superiority to CT angiography and SPECT imaging, flurpiridaz F-18 also holds advantages over other current PET tracers, namely rubidium-82 and nitrogen-13 ammonia, which have shorter half-lives.
“Because of F-18 labeling, commercial cyclotrons can produce it and then deliver it to the imaging centers,” he said.
Future uses of flurpiridaz F-18 PET could include the detection of heart disease in patients with diabetes or hypertension, given its additional ability to measure myocardial blood flow, Maddahi added.
“I think that this could be used as a way of detecting the extent of abnormalities in these patients and evaluating how treatment of diabetes or treatment of hypertension increases myocardial blood flow,” he said.
Click below to listen to the full interview.
AuntMinnie.com · Interview with Jamshid Maddahi, MD, on the impact of F-18 flurpiridaz