CHICAGO - Philips Healthcare of Andover, MA, is launching a new line of MRI scanners at this week's RSNA show. The company is also launching a new 128-slice CT scanner and PET/CT system, and is showing a hybrid PET/MRI scanner.
MRI
The company's new MRI family, called Ingenia, includes scanners at the 1.5-tesla and 3-tesla field strengths. The line features a new concept in signal transmission called digital broadband MR: Signals are digitized immediately at the radiofrequency (RF) coil and transmitted to the reconstruction engine via fiber-optic cable. This ensures the delivery of high-quality data without the signal loss that can occur on conventional MRI scanners, where signals travel through cables until they reach the analog-to-digital converter several feet away.
Ingenia's digital broadband MR concept results in a 40% increase in signal-to-noise ratio, according to the company. Another advantage is that it results in a channel-independent architecture for RF coils -- Ingenia scanners are currently rated for up to 128-channel coils, but Philips will be able to introduce coils with an even greater number of channels that will work on existing Ingenia systems, the company said.
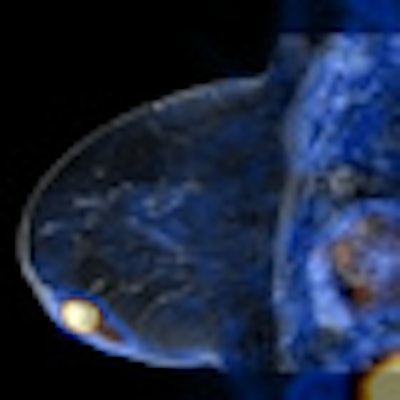
 |
| MR image from Ingenia, courtesy of Philips. |
Lastly, the Ingenia product line features low power consumption, which should pay off with lower energy costs at sites where the scanners are installed. Philips also claims that the scanners can be installed more quickly than other systems, with a nine-day average installation time.
Ingenia MRI scanners are operating at four beta sites in Europe and the U.S. Philips has filed a 510(k) application for the systems with the U.S. Food and Drug Administration (FDA) and was awaiting final clearance during RSNA week.
CT
Philips is also launching a new product in CT, called Ingenuity CT. The system is a 128-channel scanner that represents the company's effort to maximize both dose reduction and image quality. Philips has made improvements to the scanner's digital acquisition system that lowers signal loss and improves image quality.
Ingenuity CT also features SyncRight, technology that integrates the CT scanner with a contrast injector from Medrad of Indianola, PA, which eliminates the need for extra equipment. Other equipment enhancements include a new x-ray generator, new patient table, and faster reconstruction engine. The scanner also supports patient-specific imaging protocols that can reduce injected contrast dose by 15% by reducing the amount of contrast delivered after procedures are finished.
In other new CT developments, Philips is highlighting iDose 4, the newest version of the company's iDose iterative reconstruction protocol for reducing dose and improving image quality. Reconstruction time has also been reduced to two minutes. Philips has more than 100 orders in hand for iDose 4, and deliveries are expected to begin in the first quarter of 2011.
Nuclear medicine
 |
| Philips is highlighting its work-in-progress PET/MRI scanner. |
PET/MRI is one of the hottest areas in molecular imaging, and Philips discussed its work in this area at the European Congress of Radiology (ECR) in March. This week at RSNA, the company is showing an actual system gantry and is discussing its plans for commercialization of the technology.
Rather than insert a PET detector array inside a 3-tesla MRI gantry, the Philips approach uses separate MRI and PET gantries with a shared turntable-style patient table in between. The patient is shuttled between the scanners and images are fused during reconstruction. Philips believes this approach minimizes image artifacts that can develop with the PET insert approach.
Philips believes that PET/MRI will demonstrate its value in a number of clinical applications, including areas such as the prostate and breast, which have been more difficult to image with PET/CT. PET/MRI scans are performed with the company's time-of-flight protocols, and MR images are used for attenuation correction as well.
Philips is demonstrating on the RSNA show floor the model it has installed at three beta sites (only a panel display of the scanner was used at the ECR meeting). A 510(k) application is pending for the system, which can also operate as a standard 3-tesla MRI scanner. This latter point is key, as PET/MRI is currently not reimbursable in the U.S., so users can continue to use the hybrid system for separate MRI and PET studies.
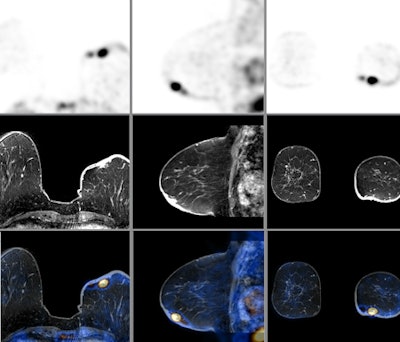 |
| PET, MR, and fused PET/MR images of a breast cancer patient. Images courtesy of the University of Geneva. |
Healthcare informatics
At the show, Philips has launched a multimodality, multivendor image viewing tool called IntelliSpace Portal, which allows radiologists to remotely view CT, MR, and nuclear medicine images. The software is based on thin-client architecture and offers users the ability to view images anywhere without having to move to a specialized workstation. It features Collaborator, a medical networking platform that allows radiologists, referring physicians, and specialists to share images and data and to discuss cases in real-time via live chat. IntelliSpace Portal can be integrated with Philips' PACS or those of other vendors, and it can be used for radiology, cardiology, oncology, and other specialties.
Philips is also highlighting a series of five new workflow layer applications for its iSite PACS software. The applications facilitate compliance with American College of Radiology (ACR) and Joint Commission standards, in addition to boosting productivity, collaboration between departments, and the efficiency of patient care.
Ambient Experience
At this year's RSNA meeting, Philips is highlighting its newest application of Ambient Experience: radiography. The company completed its first installation of the package in April at Nemours Children's Clinic in Jacksonville, FL.
Philips' Ambient Experience technology allows patients to select one of 10 different themes expressed in lighting, visuals, and sound for the imaging room using a touchscreen tablet. Potential themes include butterflies, scenes of Europe, rolling hills, or, for pediatric patients, jungle themes or undersea animation; patients can begin to interact with the technology in the waiting room. In addition to radiography settings, Ambient Experience technology can be used in MRI, CT, PET/CT, mammography, catheterization labs, emergency clinics, and interventional suites.
Philips is also showcasing its Healing Environment service. The vendor helps facilities develop a plan for the imaging department from construction to completion -- or at any point in between -- that works for both patients and staff.
X-ray
In x-ray, Philips is touting Juno DRF, a new remote-controlled fluoroscopy unit that also performs radiography. With the number of fluoroscopy procedures being performed declining, Philips saw an opportunity to combine two types of systems into one unit. Juno DRF users can conduct fluoroscopy procedures in the morning and conventional radiography in the afternoon, according to the company.
The system includes a high-end 17 x17-inch flat-panel digital radiography detector, and stitching for orthopedic exams is available. On the fluoroscopy side, Juno DRF can perform digital subtraction angiography (DSA), supports speeds up to 30 frames per second, has a source-to-image distance (SID) of 180 cm, and has a table weight capacity of up to 284 kg (626 lb).
Another new introduction is DigitalDiagnost - ER Wireless, a slimmed-down version of the company's DigitalDiagnost radiography system for emergency departments. The configuration consists of just three components: a patient table, tube stand, and workstation with the company's Eleva user interface.
Philips designed the system to be easy to handle and to reduce interference with life-support equipment found in a typical ER, such as patient monitors. The company is also touting the fact that its lack of a cable minimizes the risk of infection in the ER.
Both Juno DRF and DigitalDiagnost - ER Wireless have 510(k) clearance; Juno DRF began shipping in October with the first installation at Yuma Regional Medical Center in Yuma, AZ. Shipments of DigitalDiagnost - ER Wireless are scheduled to begin in the second quarter of 2011.
Philips is also touting a new package for pediatric imaging called Pediatric Extremity IQ. The package is designed to improve contrast resolution in small pediatric body parts by 45% -- with no increase in radiation dose -- through a reduction in kV settings. The package will be offered starting in the second quarter.
By Brian Casey and Kate Madden Yee
AuntMinnie.com staff writers
November 29, 2010
Related Reading
Philips wins Fla. x-ray install, November 4, 2010
Philips, ISRRT target dose reduction, November 2, 2010
Philips, Electron install Russian CT scanner, November 1, 2010
Philips launches iU22 Matrix US scanner, October 26, 2010
Philips outlines RSNA product plans, October 20, 2010
Copyright © 2010 AuntMinnie.com




















