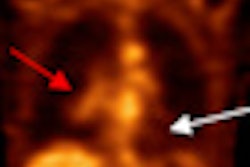
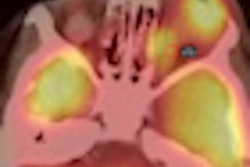
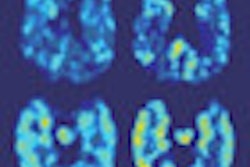
Radiopharmaceutical developer Cell>Point has signed a licensing agreement for South Korea, Taiwan, Malaysia, Vietnam, and the Philippines with Hyun IMC, headquartered in Seoul.
The license agreement covers the kit manufacture, marketing, and distribution of Cell>Point's technetium-99m-labeled glucosamine (Tc-99m EC-G) cancer and cardiology imaging product.
In addition, Hyun IMC will be responsible for sponsoring clinical studies and seeking regulatory approval in each of the countries covered by the license.
Cell>Point will receive an upfront payment and development and regulatory milestone payments, as well as royalties on gross sales from Hyun IMC.