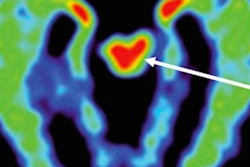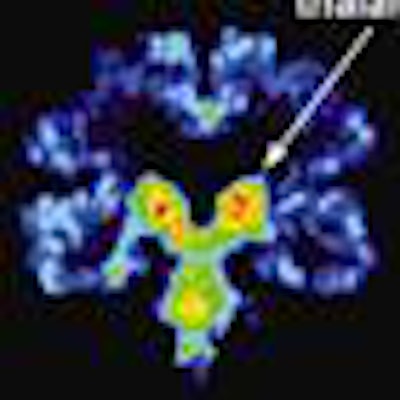
For the first time, using the radiopharmaceutical FDDNP with PET, researchers at the University of California, Los Angeles (UCLA) have identified abnormal tau proteins associated with repetitive head injury in five former professional football players, according to a study in the American Journal of Geriatric Psychiatry.
The preliminary findings from the retired National Football League (NFL) players, who had histories of mood and cognitive symptoms, confirm the presence of the protein, which has been associated with Alzheimer's disease and previously could be identified only through autopsy.
The early detection of tau proteins may help healthcare providers understand what's happening sooner in the brains of injured athletes, said lead study author Dr. Gary Small, Parlow-Solomon Professor on Aging and a professor of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience and Human Behavior at UCLA.
The findings may also help determine strategies and interventions to protect people with early concussion symptoms, rather than treating them when their conditions become more extensive (Am J Geriatr Psychiatry, February 2013, Vol. 21:2, pp. 138-144).
Brain injuries
The issue of brain trauma in athletes from continuous blows to the head has come to the forefront in recent months, highlighted perhaps most notably by the May 2012 death of former linebacker Junior Seau, who committed suicide at the age of 43. Follow-up studies determined that Seau suffered from chronic traumatic encephalopathy (CTE), a degenerative condition caused by a buildup of tau protein. CTE has been associated with memory loss, confusion, progressive dementia, depression, suicidal behavior, personality changes, abnormal gait, and tremors.
According to the U.S. Centers for Disease Control and Prevention (CDC), there are an estimated 1.6 million to 3.8 million sports-related traumatic brain injuries annually, including cases that are never reported. While most of those injuries are minor concussions, many incidents are repeat injuries and subconcussive blows to the head, the agency noted.
Last September, the NFL announced it would contribute $30 million in funding to the Foundation for the National Institutes of Health, with CTE and concussion management and treatment among the research targets.
FDDNP-PET research
UCLA's Small and colleagues are credited with developing FDDNP-PET imaging to measure both tau tangle and amyloid plaque deposition in the brains of living people (Am J Geriatr Psychiatry, Winter 2002, 10:1, pp. 24-35).
Subsequent research by the group found that FDDNP signals on PET scans can differentiate Alzheimer's disease from mild cognitive impairment and normal aging, and can predict future cognitive decline in nondemented subjects (New England Journal of Medicine, December 21, 2006, Vol. 355:25, pp. 2652-2663; JAMA Neurology, February 2012, Vol. 69:2, pp. 215-222).
According to Small and colleagues, FDDNP is the only PET probe of tau that has been studied in vivo in human imaging trials. UCLA owns three U.S. patents on the FDDNP chemical marker.
In this study, the researchers recruited five retired players ranging from 45 to 73 years of age who had histories of cognitive or mood symptoms. The participants included a linebacker, quarterback, offensive guard, defensive lineman, and center, all of whom played professionally from 10 to 16 years, with a median of 14 years. Their diagnoses included mild cognitive impairment, dementia, depression, and normal aging.
The five former athletes underwent laboratory tests and CT and MRI scans to exclude other possible causes for their mental symptoms, such as stroke or tumor. The images were also used for coregistration with PET scans (ECAT HR+ PET or Biograph PET/CT, Siemens Healthcare) for region-of-interest analyses. For the PET exams, the athletes were injected with 10 mCi of FDDNP.
FDDNP-PET signals in the subcortical (caudate, putamen, thalamus, subthalamus, midbrain, cerebellar white matter) and cortical regions of the brain (amygdala, frontal, parietal, posterior cingulate, medial and lateral temporal) were compared with results of five male controls of comparable age, education, and body mass index.
Clinical assessments were performed within four weeks of scanning, and clinicians were blinded to scan results.
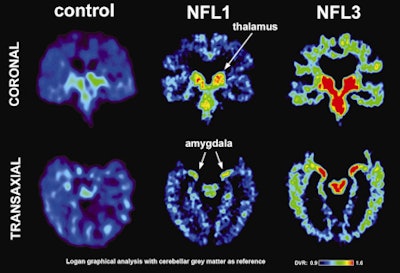 |
| Left images show normal brain scans; middle and right images show scans of football players. Green and red colors demonstrate a higher level of tau protein. Scans of the players in the study reflect differing levels of tau protein and follow a pattern of progression similar to the tau deposits that have been observed at autopsy in CTE cases. Image courtesy of David Geffen School of Medicine at UCLA. |
Elevated FDDNP
The FDDNP-PET images showed that the former NFL players had significantly greater FDDNP signals than the controls in the caudate, putamen, thalamus, subthalamus, midbrain, and cerebellar white-matter regions. The athletes and the healthy control group did not differ significantly in FDDNP binding in cortical regions except for the amygdala.
Median FDDNP signal in PET images
|
The amygdala and subcortical regions of the brain control learning, memory, behavior, emotions, and other mental and physical functions.
The review of the clinical assessments showed that the players had more depressive symptoms than the healthy group and also demonstrated cognitive loss. Three players had mild cognitive impairment, one had dementia, and one had normal cognitive function.
The results "suggest a link between the players' history of head injury and FDDNP binding," Small and colleagues concluded. "Moreover, these binding patterns (high subcortical and low cortical binding except for the amygdala) are consistent with tau deposition patterns observed in autopsy studies of CTE and differ from those observed in patients with cognitive and mood symptoms without prior head trauma, who mainly present with increased cortical FDDNP binding."
The researchers recommended larger follow-up studies to determine the effect of detecting these tau proteins early. Given the large number of people at risk for mild traumatic brain injury, such as military personnel and car accident victims, the ability to evaluate the brain during the early stages could considerably impact public health, according to the group.