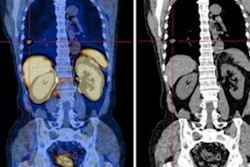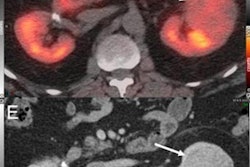
Australian researchers have developed a technique that uses a follow-up SPECT/CT scan after radionuclide therapy to personalize the radiation dose delivered, according to a study published in the July issue of the Journal of Nuclear Medicine.
The technique could improve the treatment results of patients, said lead author John Violet, PhD, of the Peter MacCallum Cancer Center in Melbourne.
"In most radionuclide therapies, a fixed amount of radionuclide is administered, which does not take into account individual patient variability in tumor and normal tissue uptake, and hence absorbed dose," he said in a statement released August 10 by the Society of Nuclear Medicine and Molecular Imaging (SNMMI). " 'Dosimetry-led' administration, on the other hand, where administered activity is adjusted based upon predictable tumor and normal tissue absorbed doses, has the potential to greatly improve therapeutic outcomes."
Using dosimetry to check patients' treatment response calls for multiple SPECT/CT scans, and therefore has not been used outside of clinical trials, Violet and colleagues noted. To address this challenge, the group developed a dose-response estimation approach from a single post-treatment SPECT/CT scan to evaluate lutetium-177-labeled prostate-specific membrane antigen (PSMA)-617 therapy for metastatic prostate cancer (J Nucl Med, July 1, 2020, Volume 61:7, pp. 1030-1036).
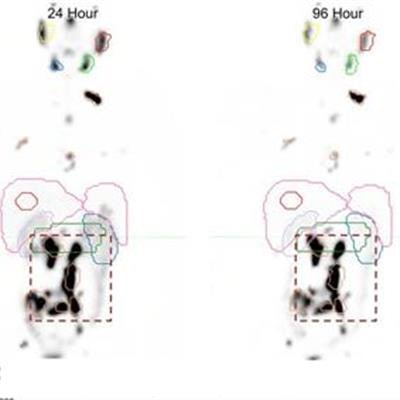
In the study, 29 patients treated with lutetium-177-labeled PSMA-617 underwent SPECT/CT imaging at four, 24, and 96 hours to track the tracer's behavior in both tumors and normal tissue. The researchers used the data from these scans to create a PSMA radiotherapeutic agent clearance model for tumors and healthy organs.
The researchers found that using the model for PSMA retention with data from the scan performed after 72 hours best allowed them to estimate a patient's radiation absorbed dose in a single image measurement.
"Tumor dose estimates were most accurate using delayed scanning at times beyond 72 hours," the group noted. "Dose to healthy tissues was best characterized by scanning patients in the first two days of treatment because of the larger degree of tracer clearance in this early phase."
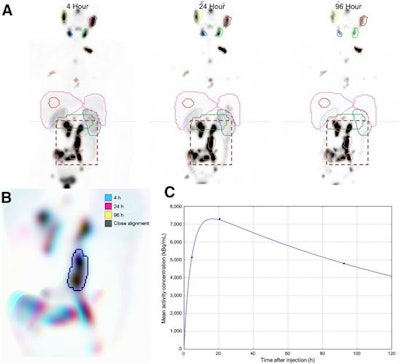 (A) Initial coarse alignment of full image volume for serial four-, 24-, and 96-h quantitative SPECT images. (B) Output of focused image registration based on contour-defined mask (dashed region of serial maximum-intensity projections in A). Three-color maximum-intensity projection is used to verify alignment for serial quantitative SPECT images, with 3D contour region highlighted. Black indicates good alignment whereas color-fringing shows areas with spatial offset. (C) Three-phase exponential curve is generated and parameters are used for subsequent population analysis. Image courtesy of SNMMI.
(A) Initial coarse alignment of full image volume for serial four-, 24-, and 96-h quantitative SPECT images. (B) Output of focused image registration based on contour-defined mask (dashed region of serial maximum-intensity projections in A). Three-color maximum-intensity projection is used to verify alignment for serial quantitative SPECT images, with 3D contour region highlighted. Black indicates good alignment whereas color-fringing shows areas with spatial offset. (C) Three-phase exponential curve is generated and parameters are used for subsequent population analysis. Image courtesy of SNMMI.This streamlined dosimetry approach would make the technique more readily available in clinical practice, allow researchers to develop models specific to particular radionuclide therapies, and offer a better understanding of radiation dose response so that physicians could tailor treatments for their patients, according to Violet.
"The routine application of radiation dosimetry would ... [provide] significant improvements in both the safety and efficacy of treatment," he said. "The adoption of routine dosimetry across molecular imaging and nuclear medicine in general would be a major advance in the field for both the clinical application of established therapies and the optimization of novel treatments."