F-18 fluoroestradiol (FES)-PET/CT can be useful in detecting clinically significant breast cancer, according to preliminary study results presented June 12 at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting in Vancouver, Canada.
Researchers from California have collected data from 60 patients thus far in an ongoing prospective clinical trial that's comparing PET/CT performed with FES -- an estrogen receptor (ER)-targeting radiotracer -- and current standard-of-care imaging methods for detecting disease in ER-positive breast cancer patients with locally advanced and suspected recurrent disease. So far, they've found FES-PET/CT identifying nodes and metastases that were missed by CT, FDG-PET/CT, ultrasound, and breast MRI.
"Breast cancer metastases in particular are often suboptimally evaluated by our current standard-of-care methodology and that limits our ability to provide optimal care," said Dr. Gary Ulaner, director of molecular imaging and therapy at the Hoag Family Cancer Institute in Newport Beach. "There is preliminary evidence that FES-PET may outperform current standard-of-care methods, such as CT bone scan and FDG-PET/CT for the detection of clinically significant and treatment-altering disease in patients with estrogen-receptor-positive breast cancer in two cohorts."
F-18 fluoroestradiol was developed by French biomarker firm Zionexa and marketed under the brand name of Cerianna. The PET radiotracer received clearance from the U.S. Food and Drug Administration (FDA) in 2020 for use in the detection of estrogen receptor-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer. In May 2021, Zionexa was acquired by GE Healthcare.
This prospective clinical trial began prior to FDA clearance, which does not include initial diagnosis as an indication for Cerianna. Thus "the value of FES-PET at initial staging and diagnosis, as well as the detection of suspected recurrence, particularly as compared with the current standard of care, is unknown," Ulaner told SNMMI attendees.
Two cohorts
The researchers created two cohorts for their study. One group of women includes newly diagnosed patients with local advanced stage 2B to 3C breast cancer and no known distant metastases. The second group consists of women with known breast cancer and who previously have been treated but now show signs of recurrence.
All patients have undergone either CT bone scans or FDG-PET/CT, followed by FES-PET. Results were evaluated separately to determine the presence of distant metastases in the first group, while the second group's images were used to discover any lesions related to a suspected recurrence. Detected lesions were biopsied, so histology and pathology could serve as the gold standard in head-to-head comparisons of FES-PET, CT bone scans, and FDG-PET/CT.
To date, 32 patients have been enrolled in the initial diagnosis and advanced cancer stage group. Of those patients, FES-PET found distant metastases in seven women (22%), which, Ulaner acknowledged, is approximately the same percentage one would expect with FDG-PET.
"While you might say this [result] looks about the same, I am actually very excited about [the number], because this [initial breast cancer diagnosis] is currently not an FDA-approved indication for using FES," he added. "The data, halfway through the trial, suggests FES performs as well as current standard-of-care modalities."
Additionally, two of 21 patients (10%) in the first group had pathologically proven axillary nodal metastases that were detected on FES PET/CT, but not seen on prior axillary ultrasound.
Among the group of 28 women with suspected cancer recurrence, the site of cancer recurrence was found in eight patients (28%), with the discovery made by FES-PET/CT alone on four women (50%). CT bone scans and FDG-PET/CT only detected distant recurrence in four of the eight patients.
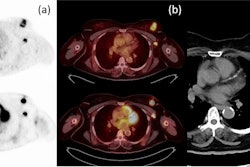
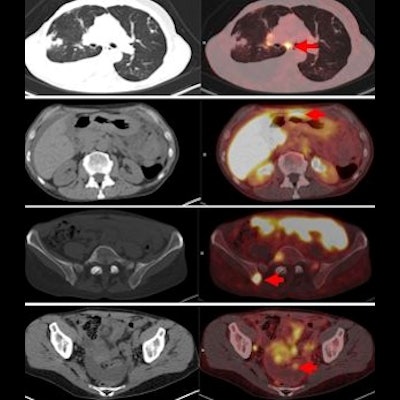
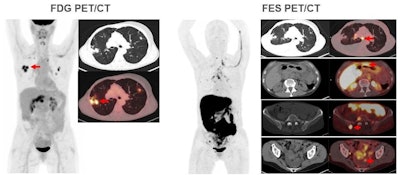 Images are from a 59-year-old woman with treated invasive lobular breast cancer and suspicion of disease recurrence. FDG-PET/CT showed FDG-avid lung opacities suspicious for lung cancer recurrence, but lung biopsy was benign. FES-PET/CT determined lung lesions were not FES-avid, but there were FES-avid nodal, gastrointestinal, osseous, and peritoneal lesions. Image courtesy of Dr. Gary Ulaner and SNMMI.
Images are from a 59-year-old woman with treated invasive lobular breast cancer and suspicion of disease recurrence. FDG-PET/CT showed FDG-avid lung opacities suspicious for lung cancer recurrence, but lung biopsy was benign. FES-PET/CT determined lung lesions were not FES-avid, but there were FES-avid nodal, gastrointestinal, osseous, and peritoneal lesions. Image courtesy of Dr. Gary Ulaner and SNMMI.Encouraging results
Ulaner cited a few limitations to the results, including that only one site of biopsy was performed in each patient and there were no biopsies if results were negative on all scans, which would preclude any data on a false-positive rate. Still, the researchers are encouraged that FES-PET/CT outperformed or matched current standard-of-care methods for the detection of cancer in both groups.
"We are finding lymph nodes that even ultrasound does not find suspicious," he emphasized, adding that it is "still not anywhere near the point where FES-PET should be used for axillary staging. That should still be [evaluated by] tissue. FES-PET/CT will not replace pathology for axillary staging, but still it is impressive how it picks up axillary nodes that are overlooked by other imaging modalities."