No pain, no gain, or so the saying goes. An MR study indicates that how much pain a person can withstand is highly individualized. In an online edition of the Proceedings of the National Academy of Sciences, international researchers offered objective proof of differences in pain sensitivity.
"Pain is a very personal, private experience that is exceptionally difficult to appreciate from a third-person perspective," said Robert Coghill, Ph.D., lead investigator and assistant professor of neurobiology and anatomy at Wake Forest University School of Medicine in Winston-Salem, NC. "Despite the increasingly widespread clinical use of a variety of pain rating techniques, there has been a lack of objective, physiological evidence that supports the validity of such pain ratings."
Coghill’s co-authors were John McHaffie, Ph.D., associate professor of neurobiology and anatomy at Wake Forest, and Ye-Fen Yen, Ph.D., from the University of Western Ontario in Canada.
In this study, the investigators placed a computer-controlled heat stimulator on the legs of 17 healthy volunteers, eight women and nine men. During a brain MRI, the heat stimulator heated a small patch of their skin to 120° F (Proceedings of the National Academy of Sciences USA, June 24, 2003).
The subjects reported a wide range of painful experiences. Using a 10-point scale, the least sensitive subject in the study rated the pain at 1, while the most sensitive subject gave the exact same pain stimulus a 9.
"Our finding that individual differences in pain-induced brain activation closely mirror individual differences in self-reports of pain (via visual analog scales) suggests the following," Coghill said. "First, people are, in fact, capable of looking into themselves, examining their experience, and then reporting it to an outside observer (i.e. physician) in a meaningful valid fashion. Second, individual differences in the experience of pain are substantial and real."
The subjects reporting greater pain from the heat stimulus also showed on MRI an increased activation of the primary somatosensory cortex (pain location and sensitivity center) and the anterior cingulate cortex (area processing the unpleasant feelings evoked by pain).
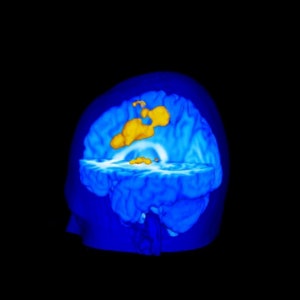 |
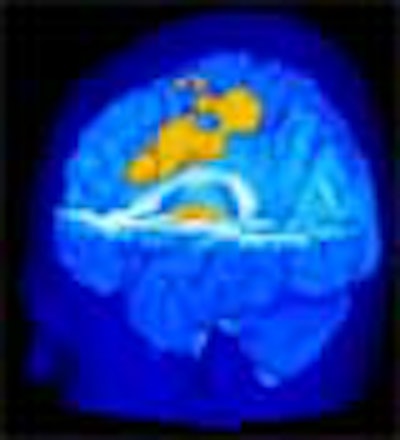
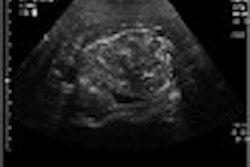
Pain-induced brain activation in highly sensitive individuals as assessed with fMRI. Note that the primary somatosensory cortex and anterior cingulate cortex had a greater magnitude of activation in sensitive individuals (above). In insensitive individuals, the thalamus displayed generally similar activation (below). Images courtesy of Robert Coghill, Ph.D.
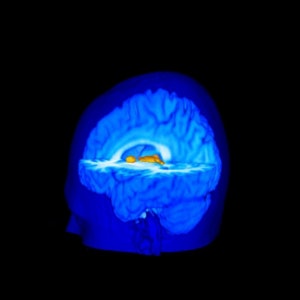 |
The investigators also found insignificant MRI variations between subjects for activation of the thalamus, which participates in pain signal transmission from the spinal cord to higher brain regions.
"This difference between cortical and thalamic patterns of activation may help explain pain differences between individuals," said Coghill. "This finding raises the intriguing possibility that incoming painful information is processed by the spinal cord in a generally similar manner. But, once the brain gets involved, the experience becomes very different from one individual to the next."
"Such differences (between subjects in response to the same pain stimulus) should be readily identified and be taken into account during a patient's treatment," Coghill said. He cited a recent Israeli study that showed a strong correlation between preoperative ratings of heat pain and postoperative ratings of pain following Cesarean section (Anesthesiology, June 2003, Vol. 98:6, pp. 1422-14266).
"This suggests that in the not too distant future, highly sensitive patients could be identified and be provided with aggressive pain management, while relatively insensitive individuals could be treated in a far more conservative fashion and spared both risk and side effects," Coghill wrote.
Coghill suggested that differences in pain sensitivity could be due to a combination of cognitive factors (like prior experience with pain) and the emotional context of a physically painful moment, as well as personal expectations about pain.
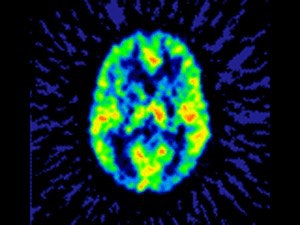 |
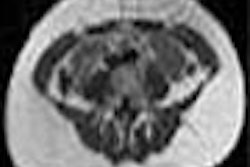
| A functional PET scan from a subject with neuropathic pain. Note the relatively low blood flow in the left portion of the thalamus, the region that receives input from the painful zone. The decreased blood flow is a consistent finding from several different types of chronic pain. Image courtesy of Robert Coghill, Ph.D. |
"One issue that we wish to emphasize is that this study does not indicate that functional imaging studies should be used to assess pain. We know that different forms of chronic pain elicit poorly understood patterns of cerebral blood-flow changes (the marker for neural activity in many functional imaging studies)," he added. "Such blood-flow changes are inconsistent with those evoked by acute pain and are inconsistent with what we know from single-neuron recording studies in animal models of pain. Thus, the self-report of pain should remain the gold standard when making treatment decisions based on the magnitude of pain."
To view QuickTime movies of this study, click here. To download the QuickTime viewer, click here.
By Bruce SylvesterAuntMinnie.com contributing writer
July 8, 2003
Related Reading
PET scans confirm that your pain really is like no other, March 12, 2003
Copyright © 2003 AuntMinnie.com


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











