With proper screening for contraindications, gadoteric acid (Gd-DOTA), a gadolinium-based MRI contrast agent, can be used safely in pediatric patients younger than 18 months old, according to a study published online July 24 in Pediatric Radiology.
Researchers at Hôpital Necker Enfants Malades in Paris found "negligible" immediate adverse effects among 104 neonates and infants younger than 18 months, and they rated image quality with Gd-DOTA (Dotarem, Guerbet) as excellent or good in 98% of the cases.
Probability of NSF
The use of gadolinium-based contrast agents for MR imaging has become very stringent in recent years, as the compounds have been associated with the potential for severe adverse side effects in patients with impaired kidney function, including nephrogenic systemic fibrosis (NSF).
Lead study author Dr. Sophie Emond, from the facility's department of pediatric radiology, and co-author Dr. Francis Brunelle noted that very few pediatric cases of NSF have been reported. "Nevertheless," the authors wrote, "there is insufficient data to suggest that NSF is less likely to occur in children than in adults with similarly significant renal disease."
Gd-DOTA is marketed in more than 70 countries and has clearance for three indications: MR imaging of intracranial and spinal disorders, whole-body imaging in adults and children, and MR angiography in adults.
Study design
The children enrolled in the nonrandomized, single-center, open-label study ranged in age from 3 days to 18 months, with a mean age of 8.1 months. There were 58 males and 45 females, with a mean weight of 8.1 kg (17.8 lb).
There were no reports of risk factors or a history of prior contrast agent reactions in any of the 104 children. Researchers did exclude children with contraindications to gadolinium.
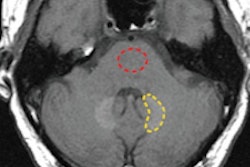
MR imaging was performed using a Signa 1.5-tesla system (GE Healthcare) with dedicated phased-array coils. MRI scans were enhanced with an intravenous bolus of Gd-DOTA at a rate of 0.2 mL/kg. The smallest amount of Gd-DOTA per child ranged from 0.6 mL in a 3-day-old newborn male to 4 mL in an 18-month-old female. The median injection of Gd-DOTA was 2 mL, followed by a saline flush of the same amount.
Children stayed in the hospital under close surveillance for at least two hours after Gd-DOTA injection. The researchers recorded any adverse reaction, the time of the event, the duration and intensity of the reaction, its likely cause, and the outcome.
The study also rated MR image quality with Gd-DOTA on a five-point scale, ranging from excellent to poor. Gd-DOTA-enhanced MR images were also rated for their diagnostic contributions from definitely normal to probably abnormal and how the scans affected a child's treatment, if at all.
In reviewing the results, the researchers found that no adverse event was reported in the 104 children after Gd-DOTA injection. "Although, in our study, NSF was not prospectively evaluated due to a short safety follow-up, no case has subsequently been brought to our attention," Emond and Brunelle wrote.
Image quality
In addition, image quality was rated as "excellent/good" for Gd-DOTA-enhanced MRI in 102 children (98%). Diagnostic contribution was also assessed as optimal (definitely abnormal/normal diagnosis) in 101 cases (97%). The use of Gd-DOTA MRI also confirmed the correct choice of initial treatment in 50 children (48%).
Possible study limitations included the small number of patients and the potential for technical difficulties when trying to scan children with MRI, the authors noted. Clinical trials with children are more challenging than those in adults, according to the researchers.
Nonetheless, "Our results suggest that immediate adverse effects are negligible following intravenous administration of Gd-DOTA in neonates and infants younger than 18 months of age who are undergoing MRI on clinical indication, and when applying the regulatory-required screening for contraindications to [gadolinium]," they concluded.
Emond and Brunelle also recommended "more extensive clinical studies" to further assess the long-term safety of Gd-DOTA with pediatric patients.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











