Women's imaging firm Hologic announced that the U.S. Food and Drug Administration (FDA) has cleared two of its MRI coils.
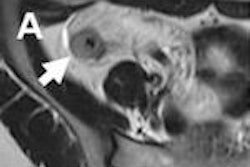
The Sentinelle MRI Endo Coil array for pelvic imaging includes Hologic technology designed to provide high signal-to-noise imaging to visualize and localize cancers in the pelvic region, especially prostate cancer, Hologic said. Siemens Healthcare and Toshiba America Medical Systems are completing final release processes to allow for product shipments in 2012.
The 16-channel Sentinelle Breast Coil array is designed to give users optimal access for breast biopsies and is pending validation by Siemens. It will initially be available on Siemens' Tim (total imaging matrix) platforms, followed by validation on Siemens' Aera and Skyra MRI platforms, Hologic said.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











