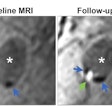
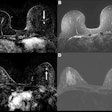
Image-guided radiotherapy developer ViewRay is highlighting clinical presentations on its MRIdian and MRIdian Linac systems at the upcoming American Society for Radiation Oncology (ASTRO) meeting in Boston.
The company's technology will be covered in 18 clinical presentations and posters from researchers at Washington University and Siteman Cancer Center at Barnes-Jewish Hospital in St. Louis; the University of California, Los Angeles Health System and Jonsson Comprehensive Cancer Center; and the University of Wisconsin Carbone Cancer Center in Madison.
ViewRay recently received CE Mark approval for MRIdian Linac; the company has also filed a 510(k) application for the device with the U.S. Food and Drug Administration (FDA).
In other ViewRay news, the company has secured a contract for MRIdian with a Tokyo-based cancer center.
The National Cancer Center has purchased the device, for which the company received marketing approval from the Japanese Ministry of Health, Labor, and Welfare in August. ViewRay is represented in Japan by Itochu, one of the three largest general trading companies in Japan, it said.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)