The U.S. Food and Drug Administration (FDA) has cleared MRI accessories developer IRadimed's MRI-compatible patient vital signs monitoring system.
IRadimed 3880 features a wireless tablet remote control that communicates vital signs information to clinicians as patients are transported from critical care departments to the MRI scanner room, according to the firm. It tracks patients' electrocardiogram (ECG), SpO2, respiratory CO2, blood pressure, and temperature data, and is rated for use in magnetic fields up to 30,000 gauss, IRadimed said.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)

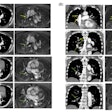




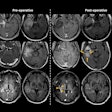
![Images depict axial MRI scans in a 50-year-old premenopausal female patient with biopsy-proven 12-mm invasive lobular cancer in the lower outer quadrant of the left breast. Upper images are subtracted dynamic contrast-enhanced (DCE) MRI scans from the first and second postcontrast acquisitions. Lower images are ultrafast MRI scans obtained within 24 hours after the DCE study (four consecutive subtracted dynamic frames from time points [T] 6–9). There is marked background parenchymal enhancement (BPE) on the DCE MRI scans. Thus, the known cancer (arrow) is barely visible; it exhibits only slightly stronger enhancement than the normal fibroglandular tissue, which results in low conspicuity. At ultrafast MRI there is less BPE, and still the cancer is barely visible because it starts to enhance simultaneously with the BPE. The conspicuity of the cancer is even reduced compared with the DCE series. Both of the readers missed this cancer on both the DCE and ultrafast MRI scans.](https://img.auntminnie.com/mindful/smg/workspaces/default/uploads/2025/05/2025-05-06-rsna-ultrafast-dce-mri-breast.Gzvq76udFr.jpg?auto=format%2Ccompress&fit=crop&h=112&q=70&w=112)

