Researchers from Massachusetts General Hospital (MGH) have had early success with a manganese-based MRI contrast agent that could become an alternative to gadolinium-based contrast agents (GBCAs), according to a study published online November 8 in Radiology.
The agent, Mn-PyC3A, has been used in animal studies for contrast enhancement of a baboon's blood vessels and was found to be equivalent to gadolinium-based agents, which carry significant health risks for some patients.
Study co-authors Peter Caravan, PhD, and Eric Gale, PhD, both from MGH's Martinos Center for Biomedical Imaging, developed Mn-PyC3A, in part, based on the element's ability to produce an MR signal comparable to that of GBCAs. In addition, the intake of small amounts of manganese is required for vital human bodily functions. The human body also can process and excrete excess manganese.
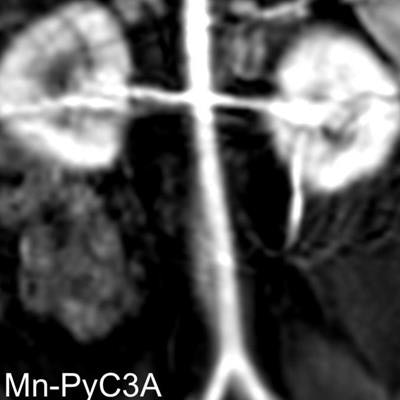
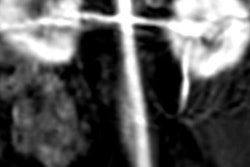
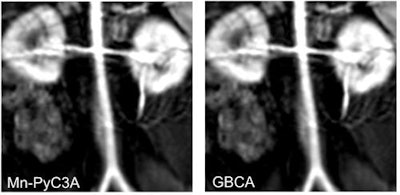 Contrast-enhanced MR image of the abdominal aorta, renal arteries, and kidneys of a baboon acquired with the manganese-based agent Mn-PyC3A (left), compared with the same scan with a gadolinium-based contrast agent (right). Images courtesy of Eric Gale, PhD.
Contrast-enhanced MR image of the abdominal aorta, renal arteries, and kidneys of a baboon acquired with the manganese-based agent Mn-PyC3A (left), compared with the same scan with a gadolinium-based contrast agent (right). Images courtesy of Eric Gale, PhD.Gadolinium, on the other hand, is not naturally found in the human body, and recent studies have raised questions about whether there are long-term adverse effects due to the metal's retention in the brain and other tissue years after a GBCA-enhanced MRI scan.
In previous studies by Caravan and Gale, mouse models showed that Mn-PyC3A was resistant to the release of manganese ions and provided good enhancement of the blood vessels, liver, and kidneys. More than 99% of the magnesium was excreted from the body within 24 hours, reducing the likelihood of prolonged retention in subjects with poor kidney function.
In the current study, the researchers compared Mn-PyC3A with a commonly used GBCA in a baboon model. Each animal underwent two MRI scans under identical conditions -- one with Mn-PyC3A and one with the GBCA. The dosages and imaging protocols were the same as those used for human patients.
As in the mouse study, Mn-PyC3A was quickly excreted through both kidney and liver clearance, leaving no evidence of the release of free manganese.
"While we did not test it here, we believe that having an alternative route of elimination through the liver will provide an efficient mechanism for elimination of Mn-PyC3A in patients with kidney disease and prevent any retention of the contrast agent in the body," Gale said. "Our next steps are to manufacture Mn-PyC3A on a larger scale and conduct additional preclinical safety studies before we can begin testing in human patients."
The two researchers created the start-up company Reveal Pharmaceuticals, which has an option to license the development of Mn-PyC3A for human use.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)