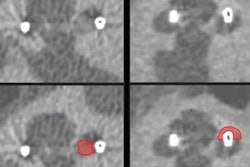
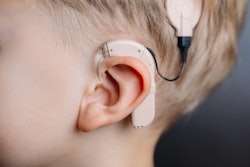
Cochlear Limited has received U.S. Food and Drug Administration (FDA) clearance for use of its Nucleus Profile Plus cochlear implant in 1.5- and 3-tesla MRI systems.
The Nucleus Profile Plus allows patients to undergo an MRI exam without the need to remove the implant's internal magnet or use a head wrap, Cochlear Limited said.
The FDA has also cleared Cochlear Limited's Nucleus 7 sound processor for cochlear implants. The device enables direct audio streaming from Apple and Android smartphones without attaching any additional intermediary devices.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











