CHICAGO - Philips Healthcare is highlighting a number of new technologies in its booth at RSNA 2014, including spectral CT and a new 1.5-tesla MRI scanner, as well as advancements in ultrasound, molecular imaging, and interventional x-ray.
CT
In a case of fortuitous timing, Philips received U.S. Food and Drug Administration (FDA) regulatory clearance just days before the meeting for IQon, the company's dedicated spectral CT scanner, which was first introduced at RSNA 2013.
IQon analyzes signals from two x-ray energies to determine changes in tissue composition. Clinicians can view the conventional grayscale anatomical images along with the spectral information from the same scan, according to the vendor. Philips estimates that IQon will begin shipping in the second half of 2015, while spectral imaging for the vendor's older iCT scanner will be available in 2016.
 The DoseWise Portal integrates dose tracking for patients and staff.
The DoseWise Portal integrates dose tracking for patients and staff.Radiation dose management has become a major issue in radiology, and Philips is stepping up to the challenge by creating an entire new business line, called DoseWise Solutions, dedicated to controlling and managing radiation dose. At the center of the business is its DoseWise Portal software for dose tracking.
The software includes a DoseAware personal dose monitoring system that measures and displays an individual's exposure to radiation in real-time. In addition, DoseAware Xtend makes real-time dose measurement even more precise and useful in a single display, giving immediate feedback on scattered x-ray dose per procedure to help staff manage exposure.
Philips believes that DoseWise Portal is unique in that it simultaneously integrates dose tracking of both patients and staff, showing all the scanning protocols that are being used and their radiation dose burden. It includes a dashboard for tracking dose from different scans, and it enables users to set their own dose reference levels (DRLs) for following dose over time.
The software is vendor-agnostic and can be used for multiple types of imaging modalities, including CT, mammography, fluoroscopy, and radiography. DoseWise Portal will begin shipping in the first quarter of 2015.
Finally, Philips plans to begin offering a suite of products and consulting services to help imaging sites set up CT lung cancer screening programs. The offering includes software tools that allow sites to follow customized clinical protocols to manage patients, lung image review and reporting applications for serial CT lung exams, and physician education resources. The program should be available in the first half of 2015.
MRI
New in the MRI section of Philips' booth is Ingenia 1.5T S, a 1.5-tesla scanner the company is launching this week. Philips developed a number of technologies to make scanning patients in the system more comfortable.
For example, a new in-bore solution provides videos to distract patients during scans, while the company's ComforTone technology reduces noise by 80%. Philips also developed AutoVoice to provide instructions and coach patients through scans, while a communications toolbox feature allows different scanner users within an enterprise to collaborate with each other on issues such as scan protocols.
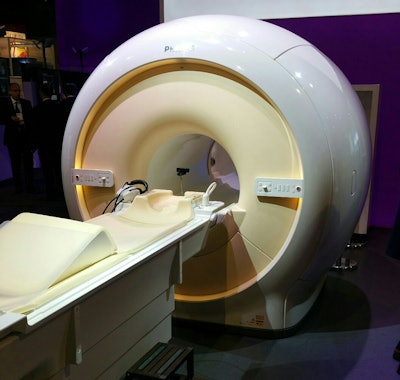 Philips' new Ingenia 1.5T S scanner.
Philips' new Ingenia 1.5T S scanner.The scanner uses Philips dStream digital broadband architecture and Omega gradients, with a maximum amplitude of 33 mT/m and a slew rate of 120 mT/m/msec. Ingenia 1.5T S will begin shipping in the first half of 2015.
In new clinical developments, Philips is demonstrating MR elastography for the liver, as well as T1, T2, and T2* mapping for cardiac studies. The company has also expanded its mDixon protocol to whole-body imaging, and it has a new application for vascular studies.
Molecular imaging
Philips is highlighting its Vereos PET/CT scanner, which it introduced at last year's RSNA show. The company replaced the photomultiplier tubes (PMTs) previously used in conventional PET cameras with digital detectors, and the system uses what Philips calls digital photon-counting technology, which converts scintillation light directly to a digital signal.
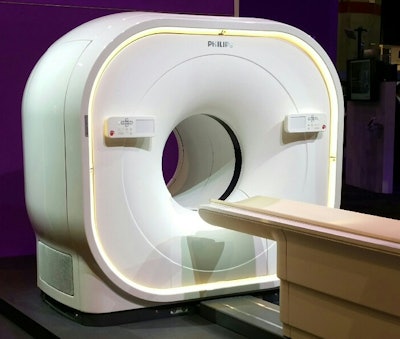 Philips is showing its Vereos PET/CT scanner.
Philips is showing its Vereos PET/CT scanner.Small crystals with one-to-one coupling to light sensors produce a high linear count rate, faster time-of-flight (TOF) performance, and overall sensitivity gains compared with the Gemini TF 16 PET/CT system. This results in twice the volumetric resolution, sensitivity, and quantitative accuracy, according to the firm.
Vereos has received 510(k) clearance from the FDA. The first system has been installed at Ohio State University (OSU) as part of a collaboration between OSU's Wright Center of Innovation in Biomedical Imaging, Cardinal Health, and the Ohio Third Frontier Commission to establish a molecular imaging pharmaceuticals center, Philips said.
Ultrasound
On the ultrasound side, Philips is highlighting its Affiniti system, which debuted at the European Society of Cardiology (ESC) congress in Barcelona, Spain, in September. Affiniti is built on the same architecture as the company's Epiq ultrasound system. It includes the firm's PureWave transducer technology, which reduces the need for image adjustment for technically difficult patients, and its Anatomical Intelligence tool, which offers automatic anatomy recognition and quantification.
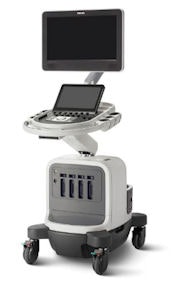 Philips is highlighting its Affiniti ultrasound system.
Philips is highlighting its Affiniti ultrasound system.Other Affiniti features include AutoScan, which automatically identifies tissue type and adjusts image gain while scanning; Auto Doppler, which provides automatic sample volume placement, spectral Doppler optimization, angle correction, and color optimization; and SmartExam, which reduces keystrokes and decreases exam time by up to 50% by automatically planning and processing application protocols, according to Philips.
The company is particularly proud of the device's ergonomic and ease-of-use features. It is 16% lighter and 60% more energy efficient than its predecessor, HD15, and it has the largest monitor in its class, Philips said. Affiniti can be rotated 180° for users to stand or sit during procedures, and it boasts a touchscreen user interface.
Philips received the CE Mark for Affiniti in September and has begun shipping the device to customers in Asia and Europe. The company plans to begin marketing the system in the U.S. in the second quarter of 2015.
In other ultrasound developments, Philips announced the creation of a consortium of healthcare leaders to help the company develop mobile ultrasound products. The consortium will focus on the development of app-based ultrasound solutions designed to interface with the company's HealthSuite Digital Platform.
Women's imaging
At this year's meeting, Philips is once again highlighting its MicroDose full-field digital mammography unit with single-shot spectral imaging (SI) technology, now augmented by the company's Spectral Breast Density Measurement package, which was cleared by the FDA last December. The spectral package allows users to gather additional spectral data at the same time as a routine mammogram, with no extra exam time or dose and no need for contrast.
Philips also plans to leverage its MicroDose SI system for 3D mammography: The device is designed to be the platform for future advanced applications such as spectral tomosynthesis, the company said.
In terms of MicroDose SI distribution, Philips inked a deal with Gamma Medica on November 24, which gives that firm the ability to offer MicroDose as a complement to its LumaGem molecular breast imaging (MBI) system. The agreement allows Gamma Medica to provide breast imaging suites with the option to purchase both technologies; Philips will continue selling MicroDose SI directly to its own customers, the company said.
Philips is also highlighting its IntelliSpace Breast workstation, a multimodality workstation that integrates mammography, ultrasound, and MRI studies so that clinicians can get a comprehensive picture of multiple breast exams from a single workspace. IntelliSpace Breast also incorporates BI-RADS standard reporting for mammography, ultrasound, and MRI to provide consistency.
X-ray and interventional
Philips is discussing its suite-based approach to interventional x-ray, with a collection of features being bundled together.
For example, the company's third-generation 16-bit detectors are being used, and Philips is offering interventional rooms in dedicated configurations called OncoSuite, NeuroSuite, and HybridSuite.
On the clinical application side, EmboGuide is a tumor segmentation protocol that overlays live fluoro to assist in the ablation of liver cancers. FDA 510(k) clearance is pending for the technique. Another technique, XperGuide Ablation, can perform overlays on PET/CT scans.
Finally, Philips announced that it has signed an agreement with Image Stream Medical (ISM) that covers the integration of its hybrid suite and interventional lab solutions with ISM's integrated video and live streaming. As part of the agreement, Philips has acquired a minority stake in ISM. Financial terms of the agreement were not disclosed.
ISM offers products and services for controlling, routing, capturing, and managing data and images utilized in various procedural environments, including hybrid suites and interventional labs. Through the deal, Philips will offer ISM's technology as a complement to its live image-guided products, clinical informatics, and services.
Imaging informatics
The big news in the imaging informatics section of Philips' booth is the launch of IntelliSpace Portal 7.0. The offering provides radiologists with a more integrated view of each patient moving along the healthcare continuum, and it enables faster delivery of diagnoses to referring physicians, the company said.
IntelliSpace Portal 7.0 connects with modality scanners and HIS, RIS, and PACS networks, allowing clinicians to review scans from virtually any location. The offering includes IntelliSpace Portal Enterprise for multisite locations.
Other new features of the 7.0 release include advanced vessel analysis, integration with Philips' Allura interventional suite to bring advanced image analysis to the point of care, and new applications for tracking and measuring chronic obstructive pulmonary disease.



















