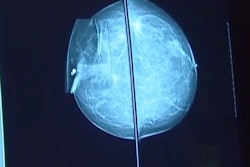
Nearly four in 10 cases of screen-detected and interval cancers had the highest AI risk score at prior screening, suggest findings published October 17 in Radiology.
Researchers led by Marthe Larsen from the Cancer Registry of Norway in Oslo also found that nearly one in four screen-detected cancers with AI scores available for two screening rounds had the highest score before diagnosis.
“This indicates a potential of AI to detect breast cancer earlier, which could lead to less harmful treatment for the affected female patients,” the Larsen team wrote.
AI continues to show its potential in mammographic screening as a standalone method for triaging exams and an assistant to interpreting radiologists. While some previous studies have reviewed AI risk scores on interval cancers, the researchers pointed out that few studies have evaluated these risk scores on prior mammograms of screen-detected cancers.
Larsen and colleagues assessed imaging data from BreastScreen Norway and AI scores on mammograms from screening exams prior to breast cancer diagnosis. They included data from 2,787 prior screening mammograms from 1,602 women with an average age of 59 years.
The team used a commercially available AI system (Transpara version 1.7.0, ScreenPoint Medical) for the study. The scores issued by the system included the following: 1 to 7, low risk of malignancy; 8 to 9, intermediate risk; and 10, high risk of malignancy.
The researchers found that 389 of the screen-detected breast cancers (38.3%) and 231 of the interval cancers (39.4%) had an AI risk score of 10 on mammograms in the screening round prior to diagnosis. They also found that among the screen-detected cancers with AI scores available two screening rounds (four years) before diagnosis, 23.0% (122 of 531) had a score of 10.
The team additionally reported that mammographic features were tied to AI scoring for invasive screen-detected cancers (p < 0.001). Finally, it found that density with calcifications was registered for 13.6% (43 of 317) of screen-detected cases with a score of 10 and 4.6% (15 of 322) for those with a score of 1 to 7.
The study authors suggested that based on these results, it may be important to recall women with calcifications and an AI risk score of 10 for assessment because it may lead to earlier detection of relevant cancers.
“However, the proportion of calcifications and an AI score of 10 among disease-free women also must be explored because it might influence the rate of false-positive screening results,” they added.
The authors called for review studies and prospective studies comparing the location of AI markings versus the location of cancers for better understanding.
“Furthermore, high AI score on mammograms in women who are not diagnosed with breast cancer represent a challenge that is important to consider,” they wrote.
In an accompanying editorial, Tejas Mehta, MD, from the University of Massachusetts wrote that the Larsen team’s results imply that implies that either the cancers found in the study truly developed since the prior mammogram or that the changes happening in the breast tissue cannot be perceived by either the current AI system or human vision. She added that the differences in AI and human vision are “synergistic.”
“As the science advances, focus should be directed on identifying clinically relevant cancers earlier and truly benign lesions, minimizing unnecessary follow-up and/or tissue sampling,” Mehta wrote. “It is with these factors in mind that we can harness the full potential of AI.”
The full study can be found here.